Rare driver mutations: encouraging results in small patient populations
As well as ALK fusion mutations and EGFR mutations, studies of the genetic profiles of patients with NSCLC have identified other mutations that might be used for additional targeted therapies. Among these, ROS1 and RET rearrangements both occur in 1 % to 2 % of patients with NSCLC.
Update of PROFILE 1001
Crizotinib is known to target not only ALK, but also ROS1, among others. Patients with ROS1-positive advanced NSCLC are being treated with crizotinib 250 mg twice daily in the ongoing phase I, open-label, PROFILE 1001 study. Initial findings confirm that targeting ROS1 is a viable strategy in ROS1-rearranged NSCLC [1]. In 2016, crizotinib was approved for the treatment of patients with metastatic/ advanced ROS1-positive NSCLC in the United States and Europe. Shaw et al. presented the up-dated results on safety and antitumour activity from the expansion phase of PROFILE 1001 [2]. Fifty-three patients were included in this analysis. The population contained three patients with ALK-negative NSCLC who were retrospectively determined to be ROS1-positive. Adenocarcinoma was the most common NSCLC histology (96.2 %), and the majority of patients (75.5 %) had no history of smoking.
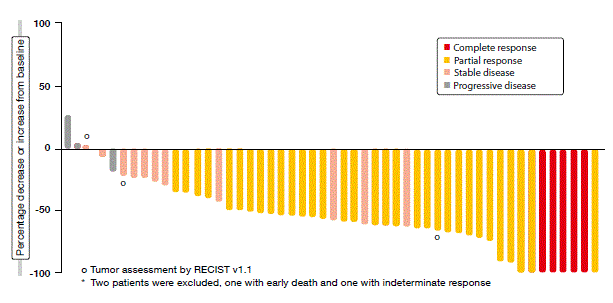
According to the analysis, the crizotinib treatment gave rise to a clinically meaningful ORR rate of 69.8 %. The patients experienced rapid responses, with median time to response of 7.9 weeks, which corresponded to the approximate time of the first on-treatment tumour scan. Responses were durable and consistent across a variety of patient demographics and baseline characteristics. Almost all of the patients had some degree of tumour shrinkage during the study (Figure). Crizotinib was generally well tolerated, with the safety profile being consistent with that observed in ALK-positive NSCLC.
Figure: PROFILE 1001: best percentage change from baseline in size of target lesions obtained with crizotinib in patients with ROS1-rearranged NSCLC*
RET-positive NSCLC: Japanese data on vandetanib
In patients with RET fusion mutations, clinical trials are underway in Japan and the United States to evaluate specific agents, including vandetanib, alectinib and cabozantinib.
Vandetanib is an oral receptor TKI that potently inhibits RET, EGFR, and VEGFR. Horiike et al. conducted a multicentre phase II trial of vandetanib 300 mg/day in patients with advanced, nonsquamous, RET-rearranged NSCLC [3]. These patients had received at least one prior chemotherapy. ORR according to the Independent Radiological Review Committee was defined as the primary endpoint. Out of 1,536 patients screened, 34 (2 %) had RET fusion. Of these, 19 patients constituted the ITT population. In this group, ORR was 47 % with vandetanib, and disease control was achieved in 90 %. Responses lasted for 5.6 months. Median PFS was 4.7 months, and 47 % of patients were alive at 1 year.
According to exploratory subgroup analyses, the type of RET fusion made a difference, as patients with CCDC6-RET (n = 6) appeared to benefit to a greater extent from vandetanib treatment than those with KIF5B-RET (n = 10), for ORR (83 % vs. 20 %, respectively), median PFS (8.3 vs. 2.9 months), and 1-year OS rate (67 % vs. 42 %). The safety profile corresponded to previous experience with vandetanib. The main AEs were hypertension, diarrhoea and acneiform rash.
The authors concluded that vandetanib showed clinical antitumor activity in patients with advanced RET-rearranged NSCLC, although large screening programmes are now required. One of these programmes is a nationwide genomic screening project called LCSCRUM-Japan. It was initiated in Japan in conjunction with this study, and it involves the identification of RET rearrangements using multiplex RT-PCR and a break-apart FISH assay. By August 2016, more than 200 institutions and 14 drug companies were participating in LC-SCRUM-Japan.
Lenvatinib in RET-positive tumours
The oral multikinase inhibitor lenvatinib targets VEGFR, FGFR and PDGFR-α, and the RET and KIT protooncogenes. In 2015, lenvatinib was approved for treatment of radioiodine-refractory, differentiated, thyroid cancer. As RET kinase is a target of lenvatinib, this appeared to be a therapeutic option for patients with RET-positive adenocarcinoma of the lung.
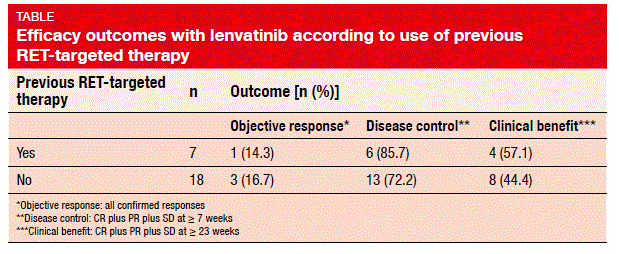
Indeed, a phase II, open-label, global, proof-of-concept study presented at the ESMO Congress showed promising clinical activity of lenvatinib 24 mg/day in 25 patients with RET-positive NSCLC [4]. The patients had received a maximum of three previous systemic therapies, with those with more than three treatments enrolled on a case-by-case basis. ORR was defined as the primary outcome, and was seen as 16 %; all of these were confirmed partial responses. Disease control (CR plus PR plus SD at ≥7 weeks) was obtained in 76 %, and clinical benefit (CR plus PR plus SD at ≥23 weeks) in 48 %. Tumour shrinkage occurred in the majority of patients. Importantly, patients showed similar ORRs, disease control rates, and clinical benefit rates irrespective of whether they had received previous RET-targeted therapy with cabozantinib or vandetanib (Table). Median PFS was 7.3 months.
For most patients, the lenvatinib toxicities were manageable with dose modifications. The most common AEs included hypertension, nausea, decreased appetite, diarrhoea, proteinuria, and vomiting. These data provide support for further studies with lenvatinib in the treatment of RET-positive adenocarcinoma of the lung.
REFERENCES
- Shaw AT et al., Crizotinib in ROS1-arranged non-small-cell lung cancer. N Engl J Med 2014; 371: 1963-1971
- Shaw AT et al., Crizotinib in advanced ROS1-rearranged non-small cell lung cancer (NSCLC): updated results from PROFILE 1001. ESMO
2016, abstract 1206PD - Horiike A et al., Phase 2 study of vandetanib in patients with advanced RET-rearranged nonsmall cell lung cancer (NSCLC). ESMO 2016, abstract 1203PD
- Velcheti V et al., Phase 2 study of lenvatinib in patients with RET fusion-positive adenocarcinoma of the lung. ESMO 2016, abstract
1204PD