EGFR-targeted treatments: insights from the adjuvant to the resistant setting
Gefitinib after complete resection
Approximately 20 % to 25 % of non–small-cell lung cancer (NSCLC) patients are eligible for surgical resection with curative intent [1]. To date, cisplatin-based chemotherapy constitutes the adjuvant standard of care for patients with stage II-IIIA completely resected NSCLC. The first-generation EGFR tyrosine kinase inhibitor (TKI) gefitinib is used as standard first-line treatment in patients with advanced EGFR-mutant NSCLC. Gefitinib showed promising results as an adjuvant strategy in the phase III ADJUVANT trial. ADJUVANT was the first prospective randomised study to compare gefitinib with vinorelbine plus cisplatin in patients with completely resected stage II-IIIA (N1/N2) EGFR-mutant NSCLC [2]. In all, 220 patients were randomised to either gefitinib 250 mg/d for 24 months or vinorelbine plus cisplatin every 3 weeks for up to 4 cycles.
For the primary endpoint, which was disease-free survival (DFS), gefitinib showed significant superiority over the chemotherapy regimen (28.7 vs. 18.0 months; HR, 0.60; p = 0.005). Three-year DFS rates were 34 % versus 27 %, and all of the subgroups favoured the TKI therapy. The adverse event (AE) profile was in keeping with that reported previously, with no cases of interstitial lung disease in the ADJUVANT trial. Significantly greater proportions of gefitinib-treated patients experienced clinically relevant improvements in health-related quality of life during the study. The authors concluded that adjuvant gefitinib could become the preferred approach in the adjuvant setting; the 2-year treatment duration for gefitinib appears to be rational and safe. For overall survival (OS), the outcomes are still immature.
Alternative first-line agent: dacomitinib
At the same time, gefitinib might be superseded as a first-line agent by the second-generation, irreversible, ErbB family inhibitor dacomitinib. The randomised, open-label, phase III ARCHER 1050 trial compared dacomitinib 45 mg/d with gefitinib 250 mg/d in 452 untreated patients with advanced NSCLC and EGFR-activating mutations [3]. Seventy-one centres in seven countries participated in this study. The majority of the patients in both arms were of Asian origin.
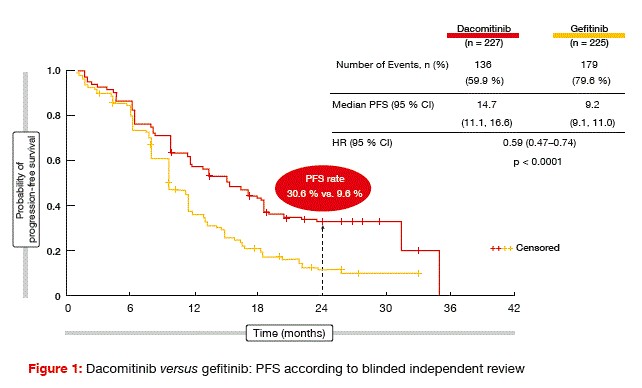
For the primary endpoint of progression-free survival (PFS) according to blinded independent review, dacomitinib outperformed gefitinib significantly (14.7 vs. 9.2 months; HR, 0.59; p < 0.0001; Figure 1). These curves only started to separate after approximately 6 months of treatment, but then they kept separating throughout the entire observation period. At 24 months, PFS rates were 30.6 % versus 9.6 %. Distinct dacomitinib-related benefits were observed for all of the subgroups, with the exception of non-Asians, which might be due in part to the smaller sample size. An exploratory analysis addressed the question of whether non-Asians who responded to treatment (n = 72) indeed performed worse than Asians. Here, the PFS curves showed the same shape as in the total population, with a HR of 0.547.
Greater depth of response in the dacomitinib arm
Objective response rates (ORRs) did not differ between the study groups, although the duration of response was significantly longer in responders who received dacomitinib (14.8 vs. 8.3 months; p < 0.0001). This discrepancy can be explained by the greater depth of response in the experimental arm.
With regard to AEs, dacomitinib treatment gave rise to higher rates of diarrhoea, paronychia, rash and stomatitis, but there were only a few more grade 3 events than in the gefitinib arm. Gefitinib, on the other hand, tended to elicit alanine transaminase (ALT) increases, with markedly higher grade 3 rates (8.5 % vs. 0.9 %). Treatment discontinuations due to AEs occurred in 9.7 % versus 6.7 % for dacomitinib and gefitinib, respectively, and dose modification rates were 66.1 % versus 8.0 %. Dose modifications of dacomitinib were possible at two dose levels, while gefitinib allowed for only one dose reduction.
Patient-reported outcomes according to EORTC-QLQ-C30 and LC13 constituted a secondary endpoint. Here, the two agents induced similar improvements in key disease-associated symptoms. The authors concluded that dacomitinib should be considered as a new treatment option for first-line management of advanced EGFR-mutated NSCLC.
Delaying acquired resistance with anti-MET treatment
Patients treated with first-generation EGFR TKIs typically develop resistance within 10 to 14 months [4, 5]. In EGFR-mutant NSCLC, the receptor tyrosine kinase MET is expressed in approximately 25 % to 75 % of cases and represents a mechanism of acquired resistance to EGFR inhibition. The bivalent MET antibody emibetuzumab was tested in a phase II study that evaluated addition of emibetuzumab to first-line erlotinib, with the purpose being to delay acquired resistance to erlotinib in EGFR-mutant, metastatic NSCLC patients [6]. Only patients who showed disease control after an 8-week erlotinib lead-in were randomised to either emibetuzumab plus erlotinib (n = 71) or erlotinib alone (n = 70).
With regard to the primary endpoint of the study, there was no PFS difference in the intention-to-treat populations (9.3 vs. 9.5 months with the combination and erlotinib, respectively). Response rates and OS did not differ, either. However, the exploratory MET biomarker analysis in- dicated a clinically meaningful PFS benefit of the combination in the subgroup of patients with the highest MET expression (≥ 90 % cells with MET 3+ staining). Here, median PFS was 20.7 versus 5.4 months with erlotinib plus emibetuzumab and erlotinib, respectively (HR, 0.39). Median OS results in this group were immature, but suggested a positive trend (not reached vs. 20.6 months; HR, 0.32). This was not explained by imbalances in baseline characteristics or molecular aberrations. AEs occurred with similar frequency in both treatment arms.
Dynamics of T790M-positive tumours
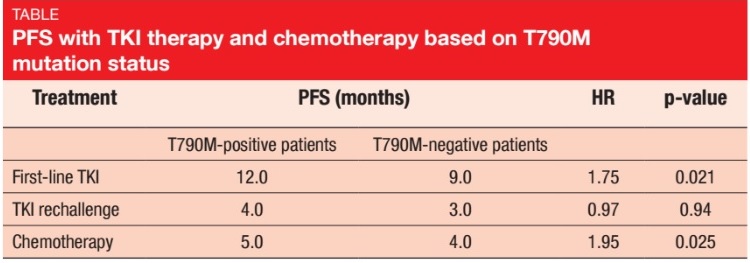
The secondary EGFR T790M mutation in exon 20 accounts for more than 50 % of acquired TKI resistance [7]. Gaut et al. examined patients with T790M mutations in terms of their response to treatment with TKIs and chemotherapy, with the aim to further characterise this important subset [8]. The patient cohort was acquired from patients who were enrolled but failed screening for the TIGER-2 and TIGER-X clinical trials that assessed the third-generation EGFR TKI rociletinib. These patients had evidence of a T790M mutation following disease progression on most recent prior EGFR- directed therapy. In the group of patients with stage IV disease at the time of treatment (n = 97), 69 patients were T790M-positive, and 28 were T790M-negative.
This study confirmed that tumours that expressed T790M had a more indolent progression of disease than their T790M-negative counterparts. PFS was superior in the T790M-positive group, for both first-line TKI therapy (12.0 vs. 9.0 months; p = 0.021; Table) and initial chemotherapy (5.0 vs. 4.0 months; p = 0.025). For TKI rechallenge, the analysis showed no statistically significant PFS difference. Response rates did not differ to any significant degree between the two groups, although there was a trend towards higher ORRs in the T790M-positive cohort in the TKI rechallenge and chemotherapy settings.
Afatinib plus bevacizumab as a successful strategy
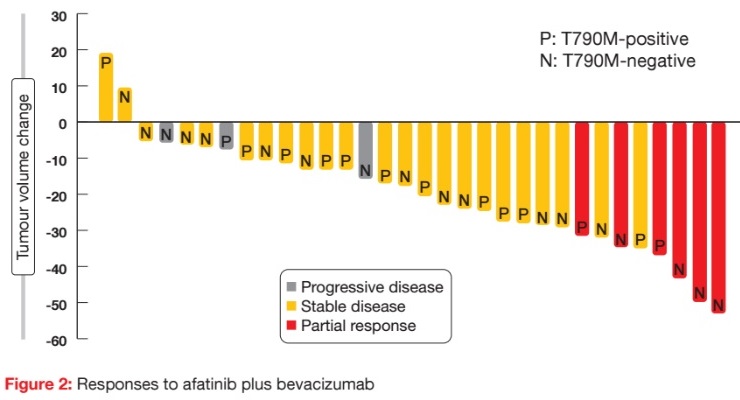
When the second-generation irreversible EGFR TKI afatinib was used as monotherapy, it had only modest activity in patients progressing on erlotinib or gefitinib [9]. However, data presented at the ASCO Congress indicated that the combination of afatinib and the VEGF antibody bevacizumab shows clinical efficacy and safety after acquired resistance to EGFR TKIs [10]. The prospective, multicentre, single-arm, phase II ABC trial evaluated the clinical efficacy and safety of afatinib 30 mg/d plus bevacizumab 15 mg/kg tri-weekly in EGFR-mutant NSCLC after acquired resistance to EGFR TKIs.
Thirty-two patients were analysed, among whom 6 (18.8 %) achieved partial responses (Figure 2). Stable disease was achieved in 71.9 %, which provided a disease control rate (DCR) of 90.7 %. Patients with both T790M-positive and T790M-negative status responded to the therapy. Median PFS was 6.3 months, and median OS had not been reached at the time of analysis. PFS did not differ across patients with T790M-positive and T790M-negative disease (6.3 vs. 7.1 months, respectively; p = 0.7910). This was also true for the comparison between tumours expressing deletion 19 and those expressing the L858R mutation (6.3 vs. 5.1 months; p = 0.7777). The authors noted that afatinib plus bevacizumab might serve as a salvage option in patients with T790M-negative tumours.
Osimertinib versus docetaxel plus bevacizumab
Patients with T790M-positive resistance to prior EGFR TKI therapy are eligible for treatment with the third-generation, CNS-active, EGFR TKI osimertinib, which irreversibly inhibits both EGFR-activating mutations and the T790M resistance mutation. An open-label, randomised, phase III trial demonstrated superiority of osimertinib over docetaxel plus bevacizumab as third-line treatment in 147 NSCLC patients whose tumours had acquired the EGFR T790M mutation. Almost all of the outcomes favoured the EGFR TKI [11]. The osimertinib-treated arm fared better regarding PFS (10.2 vs. 2.3 months; HR, 0.23; p < 0.0001), ORR (61.6 % vs. 8.3 %) and clinical benefit rate (CBR; 87.6 % vs. 43.0 %). Grade 3 or 4 toxicities occurred considerably less frequently with osimertinib than with docetaxel plus bevacizumab. The median OS had not been reached in either group. Analyses according to the EGFR mutation subtype that was present in the tumours in addition to the T790M mutation revealed that PFS and OS were both similar in patients with exon 19 deletions and L858R mutations.
ASTRIS: real-world data on osimertinib
The open-label, single-arm, multinational ASTRIS trial is the largest real-world treatment study of osimertinib to date. Osimertinib 80 mg/d is being assessed in a global population of patients with T790M-positive advanced NSCLC, who had previously received an EGFR TKI. Patients with asymptomatic, stable CNS metastases that did not require increasing doses of corticosteroids within 2 weeks were enrolled.
At the time of the interim analysis in November 2016, 1,217 patients had received at least one dose of osimertinib [12]. In the majority of patients, T790M co-occurred with one other EGFR mutation. These were mostly exon 19 deletion, L858R mutation, and exon 20 insertion. The investigator-assessed response rate was 64 % for patients evaluable for response. Twenty-nine percent had achieved stable disease. Data on OS, PFS and time to treatment discontinuation were immature. The investigators summarised that clinical activity of osimertinib resembled that observed in the clinical trial programme. No new safety signals occurred. According to preliminary safety findings, 4 % of patients had AEs leading to discontinuation of osimertinib, and 2 % had fatal AEs. Interstitial lung disease/pneumonitis-like events were reported in 2 % and QTc prolongation in 1 %. The second ASTRIS predefined interim analysis is planned for late 2017, and this will include more than 2,900 patients.
References:
- Arriagada R et al., Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two metaanalyses of individual patient data. Lancet 2010; 385: 1267-1277
- Wu YL et al., Gefitinib (G) versus vinorelbine+cisplatin (VP) as adjuvant treatment in stage II-IIIA (N1-N2) non-small-cell lung cancer (NSCLC) with EGFR-activating mutation (ADJUVANT): a randomized, Phase III trial (CTONG 1104). ASCO 2017, abstract 8500
- Mok TS et al., Dacomitinib versus gefitinib for the first-line treatment of advanced EGFR mutation positive non-small cell lung cancer (ARCHER 1050): a randomized, open-label phase III trial. ASCO 2017, abstract LBA9007
- Inoue A et al., Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol 2006; 24: 3340-3346
- Rosell R et al., Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009; 361: 958-967
- Scagliotti G et al., A randomized, controlled, open-label, phase 2 study of erlotinib with or without MET antibody emibetuzumab as first line treatment for EGFR-mutant NSCLC patients who have disease control after an 8-week lead-in treatment with erlotinib. ASCO 2017, abstract 9019
- Kuiper JL et al., Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC patients. Lung Cancer 2014; 85(1): 19-24
- Gaut D et al., Clinical implications of the T790M mutation in disease characteristics and treatment response in patients with EGFR-mutated NSCLC. ASCO 2017, abstract 9031
- Katakami N et al., LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 2013; 31(27): 3335-3341
- Hata A et al., Afatinib (Afa) plus bevacizumab (Bev) combination after acquired resistance (AR) to EGFR-tyrosine kinase inhibitors (TKIs) in EGFR-mutant non-small cell lung cancer (NSCLC): multicenter single arm phase II trial (ABC-study). ASCO 2017, abstract 9034
- Nie K et al., Osimertinib compared to docetaxel-bevacizumab as third-line treatment in EGFR T790M mutated non-small cell lung cancer. ASCO 2017, abstract 9017
- de Marinis F et al., ASTRIS, a real world treatment study of osimertinib in patients with EGFR T790M positive non-small cell lung cancer. ASCO 2017, abstract 9036