PD-(L)1 inhibition alone and in combination: recent insights into immunotherapy
First-line, single-agent pembrolizumab: KEYNOTE-042
Monotherapy with the anti-PD-1 monoclonal antibody pembrolizumab has significantly improved clinical endpoints compared to chemotherapy in patients with metastatic non–small-cell lung cancer (NSCLC) [1, 2]. KEYNOTE-024 showed overall survival (OS) improvement in addition to a progression-free survival (PFS) benefit; moreover, patients treated with pembrolizumab had a better safety profile than those receiving chemotherapy [2].
As there is an unmet need with regard to more effective and tolerable first-line regimens for metastatic NSCLC, KEYNOTE-042 investigated the role of pembrolizumab in patients with previously untreated, locally advanced or metastatic lung tumours of any histology that expressed PD-L1 (tumour proportion score [TPS] ≥ 1 %) but showed no sensitising EGFR or ALK alterations [3]. They received either pembrolizumab 200 mg every 3 weeks (Q3W) for up to 35 cycles or one of two platinum-based chemotherapy regimens for up to 6 cycles: carboplatin AUC 5 or 6 Q3W plus paclitaxel 200 mg/m2 Q3W or carboplatin AUC 5 or 6 Q3W plus pemetrexed 500 mg/m2 Q3W. There was no protocol-defined crossover.
Each arm contained 637 patients of whom almost 40 % had squamous histology. PD-L1 TPS was ≥ 50 % in nearly 50 % of cases, while approximately one third had TPS 1 % to 19 %. PD-L1 expression of 20 % to 49 % was present in approximately 17 % in each group. OS in the PD-L1 TPS subgroups of ≥ 50 %, ≥ 20 %, and ≥ 1 % constituted the primary endpoint.
Benefits related to PD-L1 expression
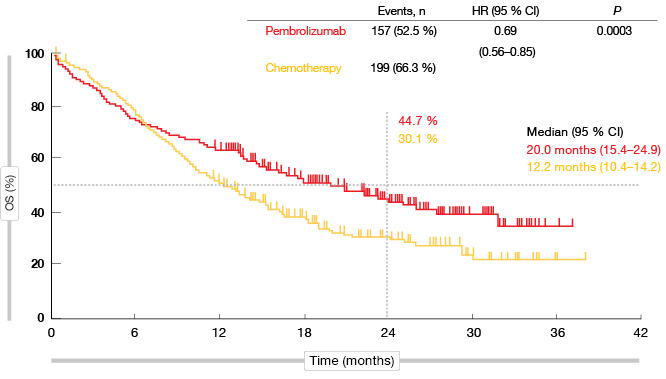
KEYNOTE-042 is the first study with a primary endpoint of OS to demonstrate superiority of pembrolizumab over platinum-based chemotherapy in the population described above. In all of the pre-defined TPS groups, the administration of pembrolizumab significantly improved survival. As for previous trials in metastatic NSCLC, the analysis yielded a greater magnitude of pembrolizumab-related benefits at higher levels of PD-L1 expression. The HRs for OS were 0.69, 0.77 and 0.81 for PD-L1 TPS ≥ 50 %, ≥ 20 %, and ≥ 1. In the TPS ≥ 50 % group, median OS was 20.0 vs. 12.2 months with pembrolizumab and chemotherapy, respectively (p = 0.0003; Figure 1). At 24 months, 44.7 % vs. 30.1 % of patients, respectively, were alive. For PFS, the difference did not meet the protocol-specified significance boundary. This outcome will be re-assessed based on additional follow-up, as the study is continuing.
Response rates did not differ to a meaningful extent between the pembrolizumab and chemotherapy arms (TPS ≥ 50 %, 39.5 % vs. 32.0 %; TPS 1 % to 49 %, 16.6 % vs. 21.7 %), although duration of response (DOR) was longer in the pembrolizumab-treated arm (20.2 vs. 8.3 months). This was true for all levels of PD-L1 expression.
Despite longer treatment exposure, treatment-related adverse events (AEs) occurred less frequently with immunotherapy than with chemotherapy. The superior safety profile suggests that pembrolizumab is an appropriate treatment option for any level of PD-L1 positivity. Overall, these data confirm and potentially extend the role of pembrolizumab monotherapy as a standard first-line treatment for patients with PD-L1–expressing tumours.
Figure 1: Overall survival obtained with pembrolizumab vs. chemotherapy in the TPS ≥ 50 % cohort of KEYNOTE-042
Assessment of pembrolizumab in squamous tumours
As add-on pembrolizumab significantly improved OS over chemotherapy alone in non-squamous NSCLC [4], evaluation in squamous tumours was a logical next step. The KEYNOTE-407 trial tested pembrolizumab 200 mg Q3W plus carboplatin AUC 6 Q3W and paclitaxel 200 mg/m2 Q3W or nab-paclitaxel 100 mg/m2 Q1W for 4 cycles compared to placebo plus the same chemotherapy regimen in untreated patients with stage IV NSCLC and squamous histology. After completion of this treatment, either pembrolizumab or placebo maintenance was administered for up to 31 cycles. The data of 278 and 281 patients who received pembrolizumab plus chemotherapy or placebo plus chemotherapy, respectively, were included in the second interim analysis, which was the first analysis of PFS and OS [5]. In both arms, approximately 35 % of patients showed a PD-L1 TPS of < 1 %, and in 37 %, TPS was 1 % to 49 %. Higher PD-L1 expression (≥ 50 %) was recorded in only 26 % of cases in each treatment arm.
The addition of pembrolizumab significantly improved OS over chemotherapy alone (15.9 vs. 11.3 months; HR, 0.64; p = 0.0008). Survival benefits occurred irrespective of PD-L1 expression, with similar HRs of approximately 60 % across all of the TPS categories. Consistently, patients receiving the checkpoint inhibitor therapy fared better with respect to PFS (6.4 vs. 4.8 months in the ITT population; HR, 0.56; p < 0.0001), objective response rates (ORR; 57.9 % vs. 38.4 %), and DOR (7.7 vs. 4.8 months). For PFS, the magnitude of benefit correlated with PD-L1 expression, with patients in the TPS ≥ 50 % group showing the highest risk reduction of 63 %.
The incidence and severity of AEs were similar in the two treatment groups, although immune-related AEs occurred more frequently in the experimental arm, which also applied to treatment discontinuations. Frequency and severity of immune-mediated AEs matched the known profile for pembrolizumab monotherapy. The authors concluded that these data suggest that pembrolizumab plus carboplatin and paclitaxel or nab-paclitaxel should become a new standard of care for first-line treatment of metastatic squamous NSCLC independent of PD-L1 expression.
Atezolizumab-based treatment for squamous NSCLC: IMpower 131
In analogy to KEYNOTE-407, the IMpower 131 trial evaluated the PD-L1 inhibitor atezolizumab plus chemotherapy compared to chemotherapy alone for 4 or 6 cycles in chemotherapy-naïve patients with stage IV NSCLC of squamous histology and any PD-L1 status. Arm A received atezolizumab together with carboplatin plus paclitaxel, while Arm B used a slightly different chemotherapy regimen (carboplatin plus nab-paclitaxel) in addition to atezolizumab. Patients randomised to Arm C (i.e., the control arm) were treated with carboplatin plus nab-paclitaxel. Maintenance therapy in the experimental arms consisted of atezolizumab, whereas Arm C received best supportive care. Each arm contained approximately 340 patients. The results presented at the ASCO Congress related exclusively to comparisons across Arms B and C [6].
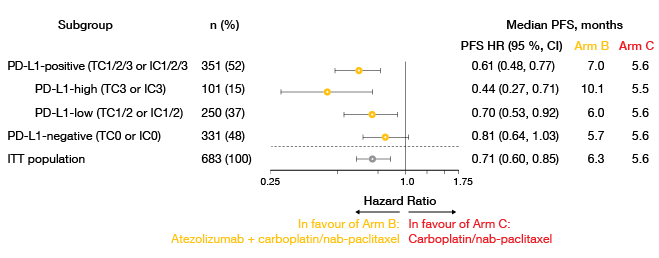
Investigator-assessed PFS in the ITT population was in favour of the atezolizumab-based combination (6.3 vs. 5.6 months; HR, 0.71; p = 0.0001). At 12 months, the PFS rate in the experimental arm was twice as high as the one in the control arm (24.7 % vs. 12.0 %). All of the pre-defined subgroups fared better with the addition of atezolizumab. This included the PD-L1 expression cohorts, even though the PFS benefit was enriched in those with higher expression (Figure 2). Similarly, responses were more pronounced in Arm B, particularly in the group expressing PD-L1 to the highest degree (ORR, 60 % vs. 33 %). DOR was longer in all of the PD-L1 subgroups, with the largest difference resulting in the PD-L1 high group (18.7 vs. 5.3 months). Most of the atezolizumab-treated patients in this cohort had ongoing responses at the time of evaluation.
The first interim OS analysis showed no difference between the two arms (14.0 vs. 13.9 months for Arms B and C, respectively). When analysed according to PD-L1 expression status, there was an OS advantage for the atezolizumab-based treatment in patients with high expression, while those with low expression fared better with the chemotherapy-only regimen. Possible causes for this are being investigated. Patients without PD-L1 expression experienced no difference between the two treatments. The safety analysis demonstrated that atezolizumab plus chemotherapy has a manageable safety profile. No new safety signals were identified. OS continues to be followed, with the next interim analysis anticipated later in 2018.
Figure 2: IMpower131: investigator-assessed progression-free survival in pre-defined PD-L1 expression subgroups
VEGF inhibition plus anti-PD-L1 activity
Immune checkpoint inhibition, chemotherapy and anti-angiogenesis are hypothesised to exert synergistic effects. For instance, the T-cell–mediated cancer cell killing brought about by atezolizumab might be enhanced by the VEGF inhibitor bevacizumab that exerts immunomodulatory effects [7].
The IMpower150 study therefore compared atezolizumab plus carboplatin and paclitaxel (Arm A) with a regimen consisting of atezolizumab, chemotherapy and bevacizumab (Arm B), and chemotherapy plus bevacizumab (control arm, Arm C). Each of the treatments was administered for 4 or 6 cycles. Maintenance therapy included atezolizumab (Arm A), atezolizumab plus bevacizumab (Arm B), or bevacizumab (Arm C). Four hundred chemotherapy-naïve patients with stage IV or recurrent metastatic non-squamous NSCLC of any PD-L1 status participated in each arm. Out of all randomised patients (ITT population), 87 % had no EGFR or ALK aberrations (ITT-WT population). Various co-primary and secondary endpoints have been defined. In 2017, the IMpower150 trial was reported as positive with respect to PFS outcomes across Arms B vs. C [8], and in March 2018, another analysis relating to OS showed positive results [9].
A new treatment standard in certain subgroups
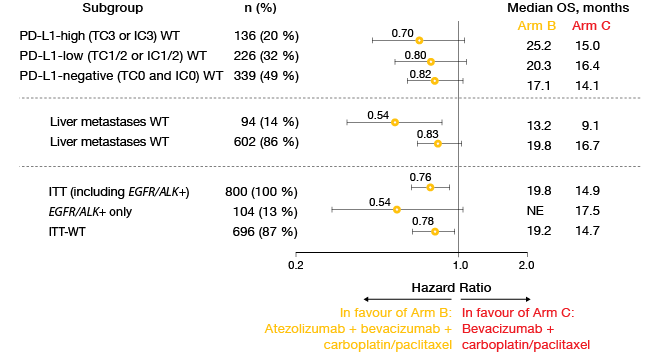
The updated PFS analysis in the ITT-WT population presented at the ASCO 2018 Congress revealed median results of 8.3 vs. 6.8 months for Arms B vs. C (HR, 0.59; p < 0.0001) [10]. At 12 months, PFS rates were 38 % vs. 20 %, and at 18 months, 27 % vs. 8 %. Likewise, the regimen featuring atezolizumab in addition to chemotherapy and bevacizumab gave rise to a significant and clinically meaningful survival benefit in the ITT-WT population, with a risk reduction of 22 % (median OS, 19.2 vs. 14.7 months; HR, 0.78; p = 0.0164). OS rates at 24 months were 43 % vs. 34 %.
Analyses of key subgroups demonstrated that the survival benefit occurred across all of the PD-L1 expression cohorts (Figure 3). Moreover, the addition of bevacizumab prolonged OS in patients with liver metastases and in all key subgroups regarding EGFR/ALK aberrations. For the OS comparison between Arm A (atezolizumab plus chemotherapy) and Arm C, a trend favouring Arm A was observed (19.4 vs. 14.7 months; HR, 0.88). This outcome will be tested again at the time of the final analysis. Further comparisons between Arms A and C in the ITT-WT population did not show any significant survival benefits in the presence of liver metastases or EGFR/ALK positivity. These results appear to be due to the interplay between the anti-VEGF treatment and the anti-PD-L1 therapy.
For ORR, Arm B outperformed the other two arms, with the highest response rate of 69 % occurring in the cohort of patients showing high PD-L1 expression. According to the authors, the data generated by the IMpower150 trial demonstrate that the combination of atezolizumab plus bevacizumab and chemotherapy provides a new standard of care, particularly for key populations studied in this trial.
Figure 3: Comparison of overall survival results in key subgroups of IMpower150 across Arms B
and C: consistent advantage due to the addition of atezolizumab
CheckMate 227: nivolumab in PD-L1–negative patients
The large, randomised, phase III, multi-part CheckMate 227 trial compared first-line nivolumab-based therapy to chemotherapy in advanced NSCLC. The co-primary endpoint was met, with nivolumab plus ipilimumab prolonging PFS in patients showing a high tumour mutational burden (TMB) of ≥ 10 mutations per megabase (mut/Mb) [11]. In patients with PD-L1 expression of < 1 %, two studies recently demonstrated that the addition of anti-PD-(L)1 therapy to chemotherapy improves outcomes compared to chemotherapy alone, with HRs for PFS of 0.75 and 0.77 [4, 12].
Therefore, an analysis of the CheckMate 227 trial presented at the ASCO Congress 2018 focused on comparing nivolumab 360 mg Q3W plus histology-based chemotherapy (n = 177) with histology-based chemotherapy alone (n = 186) in the PD-L1–negative cohort (PD-L1 expression < 1 %) [13]. Patients with both squamous and non-squamous tumours were included.
TMB as a predictor
Indeed, the PFS gain observed in the total population was consistent with the aforementioned studies (median PFS, 5.6 vs. 4.7 months for nivolumab plus chemotherapy vs. chemotherapy; HR, 0.74). At 1 year, PFS rates were 26 % and 14 %, respectively. Also, the addition of the PD-1 inhibitor led to an improvement in ORR (36.7 % vs. 23.1 %), even though responses did not appear to be more durable, with 28 % vs. 24 % of patients experiencing treatment effects beyond 1 year. The subgroup analyses suggested that the combination was particularly beneficial in patients with non-squamous histology and in those with high TMB (≥ 10 mut/Mb).
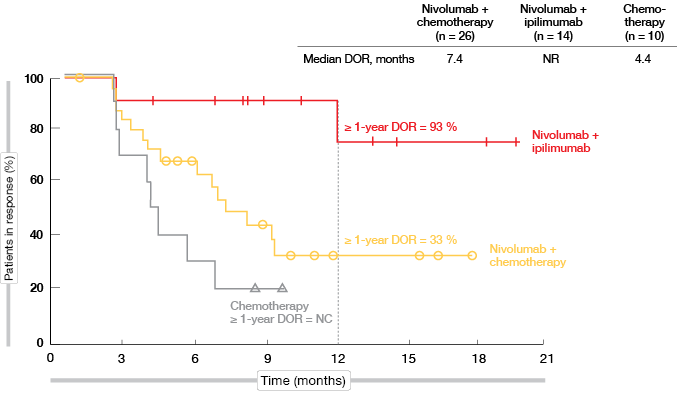
For patients with high TMB, 1-year PFS rates were 27 % vs. 8 % for nivolumab plus chemotherapy and chemotherapy, respectively (HR, 0.56). However, when juxtaposed in an exploratory analysis, the PFS obtained with the double checkpoint inhibitor regimen of nivolumab plus ipilimumab in CheckMate 227 outperformed these results (1-year PFS rate, 45 %; HR, 0.48 vs. chemotherapy). Importantly, responses in patients receiving both immune checkpoint inhibitors proved extremely durable, as median DOR had not been reached at the time of the analysis. DOR rates beyond 1 year were 93 % and 33 % for nivolumab plus ipilimumab and nivolumab plus chemotherapy, respectively (Figure 4). In patients with low TMB, on the other hand, the addition of any immunotherapy (i.e., nivolumab alone or nivolumab plus ipilimumab) did not correlate with a PFS benefit.
In their conclusions, the authors noted that TMB testing might be clinically relevant for patient selection in the PD-L1–negative group, as the PFS benefit resulting from the addition of nivolumab was enhanced in the setting of high TMB. At the same time, patients with low TMB did not appear to benefit from nivolumab in combination with either chemotherapy or ipilimumab.
Figure 4: Duration of response with nivolumab plus ipilimumab, nivolumab plus chemotherapy,
and chemotherapy only in patients with PD-L1 expression < 1 % and high TMB
Early and sustained PRO improvements
Reck et al. presented the initial patient-reported outcomes (PROs) in patients with TMB ≥ 10 mut/Mb treated with nivolumab plus ipilimumab versus chemotherapy in CheckMate 227 [14]. PROs were assessed as an exploratory endpoint using lung-cancer–specific and generic instruments including the Average Symptom Burden Index (ASBI), Lung Cancer Symptom Scale (LCSS), minimally important difference (MID), and visual analog scale (VAS). Moreover, the safety profile of the checkpoint inhibitor combination was characterised further to inform clinical practice.
The analysis showed that patients treated with nivolumab plus ipilimumab experienced rapid, durable, and clinically meaningful improvements in symptoms and overall health status. According to LCSS ASBI, these patients, while on treatment, had a longer time to deterioration in disease-related symptoms than those receiving chemotherapy (not reached vs. 6.3 months, HR, 0.43). The proportion of patients with a clinically meaningful deterioration in disease-related symptoms on or off treatment by week 12 was lower with the checkpoint inhibitor combination than with chemotherapy (22.3 % vs. 35.0 % by LCSS ASBI). Symptoms improved with nivolumab plus ipilimumab within the first 12 weeks, and the decrease in symptom burden from baseline exceeded the MID for most of the on-treatment period. With chemotherapy, on the other hand, symptoms remained similar to baseline on average. The patients‘ overall health status as per EQ-5D VAS improved with the immunotherapy regimen within the first 12 weeks and was maintained over the on-treatment period. In contrast, patients receiving chemotherapy did not experience any improvement of health status within the first 12 weeks but only after completion of 4 cycles of chemotherapy.
Toxicities observed with nivolumab plus low-dose ipilimumab across three studies (CheckMate 227, CheckMate 012, CheckMate 568; n = 941) were consistent and manageable. Treatment-related AEs gave rise to low discontinuation rates. Among patients with treatment-related select AEs, the majority of events resolved, with a median time to resolution of < 10 weeks.
NICOLAS: safety of checkpoint inhibition plus chemo-RT
The feasibility of combined chemo-radiotherapy (chemo-RT) and concurrent PD-1 inhibition is of high scientific interest. As concurrent immune checkpoint inhibition and radical thoracic radiotherapy (RT) had never been assessed in a clinical trial, the single-arm, phase II NICOLAS study was the first one to evaluate the safety of the addition of nivolumab to first-line chemo-RT in patients with unresectable, locally advanced stage IIIA/B NSCLC.
Three cycles of chemotherapy (i.e., cisplatin plus vinorelbine, cisplatin plus etoposide, or cisplatin plus pemetrexed) were administered, as well as RT with a physical dose of ≥ 60 Gy. The first four doses of nivolumab consisted of 360 mg Q3W. Thereafter, the dosage was 480 mg Q4W for up to 1 year from the start of nivolumab treatment. The pneumonitis-free rate (grade ≥ 3) observed at any time during 6 months post-RT was defined as the primary endpoint.
At the time of the interim safety analysis, which was performed in September 2017 and included 21 patients who had reached 3 months of follow-up after completion of RT, no pneumonitis grade ≥ 3 had occurred [15]. In the safety cohort followed up to February 2018 (n = 62), no unexpected AEs or increased safety risks were observed. The most frequent AEs comprised fatigue, anaemia, and nausea. The 1-year PFS will be evaluated in the expanded cohort of 74 patients in 2019.
Ongoing studies
A promising approach in tackling lung tumours with squamous histology is the combination of pembrolizumab with the ErbB family inhibitor afatinib, as EGFR overexpression is more common in squamous tumours than in adenocarcinomas. Preclinical evidence suggests that both the immune microenvironment and tumour expression of PD-L1 might be modulated by EGFR signaling in EGFR-mutant NSCLC [16, 17]. The phase II, open-label, non-randomised, single-arm LUX-Lung IO/KEYNOTE 497 study has been initiated with the aim of assessing afatinib plus pembrolizumab in patients with locally advanced or metastatic squamous NSCLC whose disease progressed during or after first-line platinum-based treatment [18]. ORR constitutes the primary endpoint.
Against the background of growing evidence suggesting that the tumour microenvironment might interfere with effective immune recognition even in the presence of checkpoint inhibitors [19], an ongoing phase I/II trial is evaluating the triple kinase inhibitor nintedanib in combination with nivolumab and ipilimumab in patients with advanced NSCLC [20]. The rationale for this study results from the fact that cancer-associated fibroblasts, which are inhibited by nintedanib, represent an important component of the tumour microenvironment and are known to promote metastasis by modifying immune cell infiltration [21]. Apart from determination of the maximum tolerated dose and the required phase II dose in the phase I part, the study aims to confirm whether concurrent nivolumab, ipilimumab and nintedanib administration is efficacious with regard to ORR in treatment-naïve and pre-treated patients.
REFERENCES
- Herbst RS et al., Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540-1550
- Reck M et al., Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823-1833
- Lopes G et al., Pembrolizumab vs platinum-based chemotherapy as first-line therapy for advanced/metastatic NSCLC with a PD-L1 TPS ≥ 1 %: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol 36, 2018 (suppl; abstr LBA4)
- Gandhi L et al., Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078-2092
- Paz-Ares L et al., KEYNOTE-407: phase 3 study of carboplatin-paclitaxel/nab-paclitaxel with or without pembrolizumab for metastatic squamous NSCLC. J Clin Oncol 36, 2018 (suppl; abstr 105)
- Jotte RM et al., Primary PFS and safety analysis of a randomised phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 36, 2018 (suppl; abstr LBA9000)
- Hegde PS et al., Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol 2017 Dec 8. pii: S1044-579X(17)30204-3
- Reck M et al., Primary PFS and safety analyses of a randomised phase III study of carboplatin + paclitaxel ± bevacizumab, with or without atezolizumab in 1L non-squamous metastatic NSCLC (IMpower150). ESMO 2017, LBA1_PR
- https://www.roche.com/media/releases/med-cor-2018-03-26.htm
- Socinski MA et al., Overall survival (OS) analysis of IMpower150, a randomised Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs chemo + bev in 1L nonsquamous (NSQ) NSCL. J Clin Oncol 36, 2018 (suppl; abstr 9002)
- Hellmann MD et al., Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093-2104
- Kowanetz M et al., IMpower150: Efficacy of atezolizumab (atezo) plus bevacizumab (bev) and chemotherapy (chemo) in 1L metastatic nonsquamous NSCLC (mNSCLC) across key subgroups. AACR 2018, abstract CT076
- Borghaei H et al., Nivolumab + ipilimumab, nivolumab + chemotherapy, and chemotherapy in chemo-naïve patients with advanced non-small cell lung cancer and < 1 % tumor PD-L1 expression: results from CheckMate 227, J Clin Oncol 36, 2018 (suppl; abstr 9001)
- Reck M et al., Nivolumab + ipilimumab vs platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: safety analysis and patient-reported outcomes from CheckMate 227. J Clin Oncol 36, 2018 (suppl; abstr 9020)
- Peters S et al., NICOLAS: safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-RT regimen in unresectable locally advanced NSCLC – the ETOP NICOLAS phase II trial. J Clin Oncol 36, 2018 (suppl; abstr 8510)
- Chen N et al., Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015; 10: 910-923
- Akbay EA et al., Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumours. Cancer Discov 2013; 3: 1355-1363
- Levy B et al., Afatinib in combination with pembrolizumab in patients with stage IIIB/IV squamous cell carcinoma of the lung. J Clin Oncol 36, 2018 (suppl; abstr TPS9117)
- Li H et al., Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 2007; 101: 805-815
- Puri S et al., Phase I/II study of nivolumab and ipilimumab combined with nintedanib in advanced NSCLC. J Clin Oncol 36, 2018 (suppl; abstr TPS9112)
- Harper J et al., Regulation of the anti-tumor immune response by cancer-associated fibroblasts. Semin Cancer Biol 2014; 25: 69-77