Antigen targets for mRNA vaccines against cancer
Introduction
Due to their crucial role in combating the SARS-CoV-2 pandemic, mRNA vaccines are currently receiving a lot of attention and, so far, have been a great success for the companies developing them. Cancer research is another major application for mRNA vaccine technology. Immuno-oncology develops ways of directing the immune system to fight against cancer cells, thus expanding the possibilities of modern oncology. Vaccines against tumor antigens are considered one of the most promising new tools in immuno-oncology. But what is the underlying fundamental principle of these vaccines?
Modern immuno-oncology explores various strategies to improve the immune system’s ability to fight cancer by activating host anti-tumor immunity and modifying the suppressive tumor microenvironment [1], resulting in tumor reduction and increased overall survival. Vaccines against oncogenic viruses, e.g. HPV and hepatitis B, are being used with great success to prevent cervical cancer and some head and neck cancers [2], [3]. Vaccination against tumor antigens is an attractive new immunotherapeutic option which carries both therapeutic and prophylactic potential. Tumor antigens are subdivided into tumor-associated antigens (TAAs), which naturally occur in different types of cells but are overexpressed in tumor cells, and tumor-specific antigens (TSAs), which are exclusive to cancerous cells. Vaccination against these antigens can induce a T-cell response against malignant cells that overexpress these antigens. Moreover, chronic therapeutic response may be achieved due to immunologic memory [4].
Vaccination against tumor-associated antigens
Tumor-associated antigens such as CD19 are already targeted by a number of immune-based cancer therapies, including monoclonal antibodies (e.g. tafasitamab) [5] and CAR-T cell therapies (e.g. tisagenlecleucel) [6]. Alternatively, cancer vaccines are being developed for active immunization against TAAs. Since TAAs are specific to entire classes of tumors, like CD19 for B-cell lymphomas, a single agent, once proven effective, could be available to many patients. On the other hand, TAAs are subject to central tolerance, which makes it difficult to induce a robust immune response. TAA vaccines also carry the risk of severe side effects due to autoimmunity. As a result, many clinical trials exploring TAA-based vaccines have disappointed with suboptimal efficacy data. Nevertheless, an effective, FDA-approved vaccine against a tumor-associated antigen has already been developed, namely, sipuleucel-T. It targets the TAA prostatic acid phosphatase, which is overexpressed in some prostatic cancer cells, particularly in metastasized cancers. Sipuleucel-T is not a conventional vaccine but contains patient-derived dendritic cells (DCs) loaded with the TAA. In a phase-III clinical trial, it demonstrated a modest benefit to patient survival.
TAA vaccines based on formulated mRNA (RNA-LPX) have recently proved to be more suitable than previous vaccine technologies to generate robust immune responses. Unlike peptide vaccines, mRNA vaccines can encode full-length antigens, which is more likely to stimulate a broad T cell response [4], [7], and their manufacturing is significantly easier than that of DC vaccines.
Vaccination against tumor-specific antigens
TSAs are considered ideal targets of immune-based cancer therapies. Since they are found exclusively in cancer cells, the immune response is not limited by central tolerance and the risk of autoimmunity is lower. Some TSAs, such as oncogenes with classic gain-of-function mutations, can occur in multiple patients and types of cancer. A number of vaccines against common TSAs are in development, such as Moderna’s mRNA-5671 (NCT03948763). The mRNA-based vaccine encodes the four most common KRAS mutations and could direct the immune system against a variety of solid tumors. BioNTech takes a different approach with BNT113, which targets the oncoproteins E6 and E7, that are introduced into cells by the tumorigenic human papillomavirus 16 (HPV16) and occurs in many head and neck cancers (NCT04534205). Both mRNA-5671 and BNT113 are also being tested in combination with pembrolizumab as studies indicate synergistic effects between cancer vaccines and checkpoint inhibitors [8]. Since TSAs are usually cancer-specific, or even patient-specific, the number of patients who can benefit from a given TSA-vaccine is generally smaller than for TAA-based vaccines. This makes clinical development more laborious and costly.
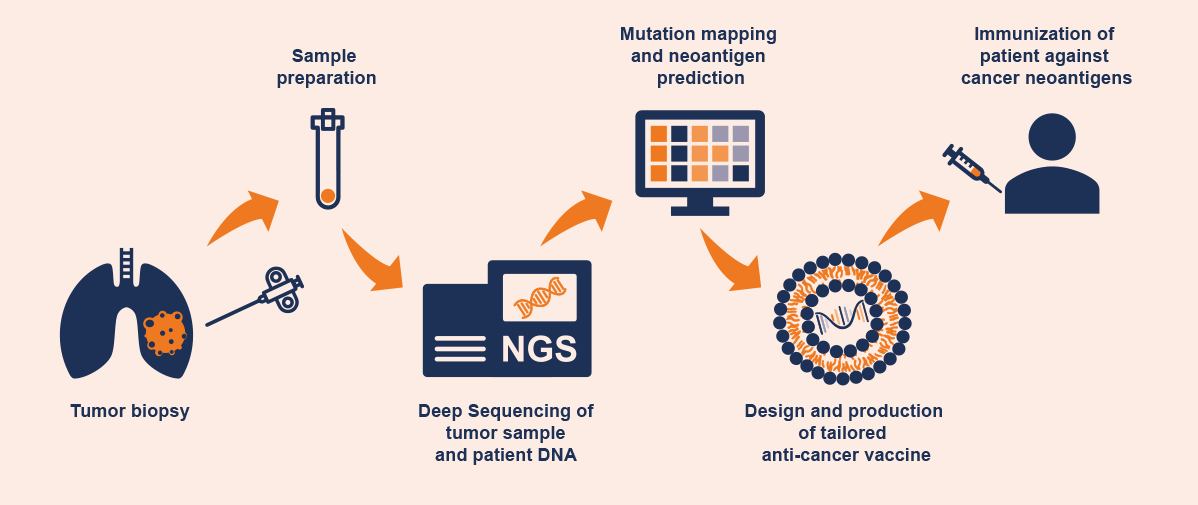
Nonetheless, the potential of neoantigen-based vaccines is enormous. With the advent of next generation sequencing, it is possible to generate a comprehensive mutation profile of a cancer by taking multiple biopsies of one or more tumors from a patient. By comparing the mutation profiles of all biopsy samples, it is even possible to create a mutation family tree and to temporally dissect the mutation hierarchy, to map tumor evolution [9], [10]. After comparing these data with the genome of healthy patient cells, suitable neoantigens can be predicted for an individually-tailored vaccine. Vaccines matched to the individual mutation profile of a patient’s tumor are already in clinical development. Examples include BioNTech’s Individualized Neoantigen Specific Immunotherapy program (iNeST, with up to 20 patient neoantigens) and Moderna’s Personalized Cancer Vaccine program (PCV, with up to 34 neoantigens). Factors including expression level, proportion of cells expressing the antigen, and the presumed immunogenicity of the antigen are considered during the process of selecting a neoantigen [11]. Thus, it is no longer the vaccine itself that is standardized, but the method of vaccine development; each patient receives a unique, customized medical product. The result is typically a multivalent vaccine designed to stimulate the patient’s immune system to develop a broad immune response against (ideally) all tumor cells.
Figure 1: Process of developing an individualized cancer vaccine
Outlook
Over the past 100 years, modern oncology has developed numerous methods to combat cancer and improve patients’ chances of survival. Owing to their ability to proliferate and mutate rapidly, cancers are able to develop resistance to radiotherapy, chemotherapy, and targeted therapies, and form a new, even more malignant cancer from a few surviving cells. For this reason, new therapeutic concepts that reach all tumor cells capable of proliferation are urgently needed. Immuno-oncology is still at an early stage of development. The FDA approval of Sipuleucel-T, the first therapeutic cancer vaccine, for the treatment of hormone-refractory prostate cancer [12] marks an important step. While its effect on patient survival is modest, it serves as a proof-of-concept for this new therapeutic approach. In addition to this initial successful attempt in cancer vaccine development, there are clinical trials with promising clinical responses in multiple solid or metastatic tumors [13], [14]. If the immune system can be armed against antigens that are important for the survival and proliferation of tumor cells, cancer would be tackled at its root, more so than with chemotherapies and approaches directed against individual key targets. Oncologic vaccine research is currently better funded than ever before – which is ideal for the potential of this new technology to be unleashed.
REFERENCES
- [1] G. K. Philips and M. Atkins, “Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies,” Int. Immunol., vol. 27, no. 1, pp. 39–46, Jan. 2015, doi: 10.1093/intimm/dxu095.
- [2] L. Cheng, Y. Wang, and J. Du, “Human papillomavirus vaccines: An updated review,” Vaccines, vol. 8, no. 3, pp. 1–15, 2020, doi: 10.3390/vaccines8030391.
- [3] M. H. Chang et al., “Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study,” J. Natl. Cancer Inst., vol. 101, no. 19, pp. 1348–1355, 2009, doi: 10.1093/jnci/djp288.
- [4] L. Miao, Y. Zhang, and L. Huang, “mRNA vaccine for cancer immunotherapy,” Molecular Cancer, vol. 20, no. 1. BioMed Central Ltd, Dec. 01, 2021, doi: 10.1186/s12943-021-01335-5.
- [5] European Medicines Agency, “Tafasitamab Profile.” https://www.ema.europa.eu/en/medicines/human/EPAR/minjuvi (accessed May 19, 2022).
- [6] European Medicines Agency, “Tisagenlecleucel Profile.” https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah (accessed May 19, 2022).
- [7] L. Li, S. P. Goedegebuure, and W. Gillanders, “Cancer vaccines: shared tumor antigens return to the spotlight,” Signal Transduct. Target. Ther., vol. 5, no. 1, Dec. 2020, doi: 10.1038/s41392-020-00364-8.
- [8] J. Zhao, Y. Chen, Z. Y. Ding, and J. Y. Liu, “Safety and efficacy of therapeutic cancer vaccines alone or in combination with immune checkpoint inhibitors in cancer treatment,” Frontiers in Pharmacology, vol. 10. Frontiers Media S.A., 2019, doi: 10.3389/fphar.2019.01184.
- [9] F. L. Fennemann, J. M. De Vries, C. G. Figdor, and M. Verdoes, “Attacking tumors from all sides: Personalized multiplex vaccines to tackle intratumor heterogeneity,” Frontiers in Immunology, vol. 10, no. MAR. Frontiers Media S.A., 2019, doi: 10.3389/fimmu.2019.00824.
- [10] C. Jolly and P. Van Loo, “Timing somatic events in the evolution of cancer,” Genome Biology, vol. 19, no. 1. BioMed Central Ltd., Jul. 24, 2018, doi: 10.1186/s13059-018-1476-3.
- [11] L. De Mattos-Arruda et al., “Neoantigen prediction and computational perspectives towards clinical benefit: recommendations from the ESMO Precision Medicine Working Group,” Annals of Oncology, vol. 31, no. 8. Elsevier Ltd, pp. 978–990, Aug. 01, 2020, doi: 10.1016/j.annonc.2020.05.008.
- [12] M. A. Cheever and C. S. Higano, “PROVENGE (sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine,” Clin. Cancer Res., vol. 17, no. 11, pp. 3520–3526, 2011, doi: 10.1158/1078-0432.CCR-10-3126.
- [13] S. M. Rittig et al., “Long-term survival correlates with immunological responses in renal cell carcinoma patients treated with mRNA-based immunotherapy,” Oncoimmunology, vol. 5, no. 5, pp. 1–8, 2016, doi: 10.1080/2162402X.2015.1108511.
- [14] A. Papachristofilou et al., “Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer,” J. Immunother. Cancer, vol. 7, no. 1, pp. 1–14, 2019, doi: 10.1186/s40425-019-0520-5.
Author: Sven Vanselow
© 2022 Springer-Verlag GmbH, Impressum