Phase III results in local and regional lung cancer
PROCLAIM: chemoradiotherapy followed by pemetrexed consolidation
The standard-of-care for inoperable stage III NSCLC is concurrent chemoradiotherapy. However, the role of consolidation chemotherapy remains controversial. The multi-targeted antifolate pemetrexed shows selective activity in non-squamous NSCLC. Choy et al. demonstrated that pemetrexed-platinum combinations can be administered at full systemic doses with concurrent thoracic radiotherapy (TRT) [1].
The randomised phase III PROCLAIM study was initiated to determine whether the concurrent administration of pemetrexed/cisplatin and TRT followed by consolidation treatment with pemetrexed provides any survival advantage compared to a commonly used chemoradiation regimen followed by a consolidation regimen of choice. Previously untreated patients with stage IIIA or IIIB non-squamous NSCLC were randomised to either pemetrexed/cisplatin plus TRT for 3 cycles (n = 301), or to etoposide/cisplatin plus TRT for 2 cycles (n = 297). This concurrent phase was followed by a recovery period lasting 3 weeks to 5 weeks.
Patients who achieved partial response, complete response or stable disease moved on to the consolidation phase. Here, the experimental arm received pemetrexed for 4 cycles (n = 229), whereas the control arm was treated with consolidation according to the investigator’s choice (etoposide/cisplatin, or vinorelbine/cisplatin, or paclitaxel/carboplatin) for 2 cycles (n = 202). The OS was defined as the primary outcome. At the ASCO Congress, the final overall results were presented [2].
Acceptable safety findings
PROCLAIM did not demonstrate superiority of the pemetrexed-based regimen with regard to OS. No differences were noted for the median outcomes (26.8 vs. 25.0 months with pemetrexed/cisplatin and etoposide/cisplatin, respectively; p = 0.831). This was also true for OS rates at 2 years (52 % each) and 3 years (40 %, 37 %, respectively). The subgroup analysis did not clearly favour any of the two regimens. For PFS, there was a trend in favour of the experimental treatment (11.4 vs. 9.8). The risk of progression was lower by 14 % in the experimental arm, but this difference was not statistically significant (p = 0.130).
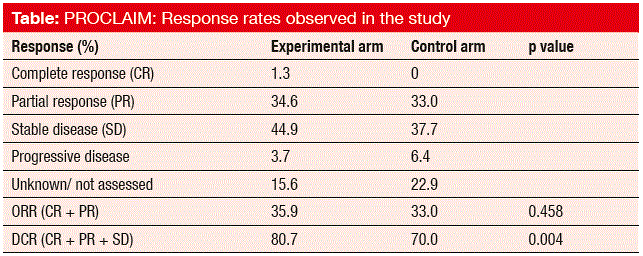
Furthermore, ORRs did not differ significantly between the two regimens (35.9 % vs. 33.0 %; p = 0.458). For DCR, however, the pemetrexed-treated patients experienced a significant benefit (80.7 % vs. 70.7 %; p = 0.004; Table), which was mostly due to a higher rate of stable disease (44.9 % vs. 37.7 %). For local relapse, both within the radiation field and inside the thorax but outside of the radiation field, there were no significant differences between the two treatment arms. This also applied to distant relapse and CNS disease.
In terms of safety, the pemetrexed-based regimen showed higher tolerability. The proportion of patients experiencing at least one drug-related grade 3 to 5 AE was markedly lower at 67.8 %, versus 79.4 % in the control arm. Administration of the control regimen was more frequently associated with neutropenia, thrombocytopenia, and alopecia. In contrast, the experimental treatment gave rise to higher rates of mucositis and stomatitis, as well as pneumonitis, which counted among the radiation-related toxicities. Grade 3/4 pneumonitis was more frequent in the control arm, however (1.8 % vs. 2.6 %). Grade 3/4 oesophagitis, as another radiation-related toxicity, was also more common in the control group (15.5 % vs. 20.6 %). The authors noted that pemetrexed/cisplatin combined with radiotherapy and followed by consolidation treatment with pemetrexed showed an acceptable safety profile.
SCAT: adjuvant chemotherapy according to BRCA1
Post-operative platinum-based chemotherapy improves outcomes in completely resected NSCLC with nodal involvement (stage II to IIIA). Survival outcomes remain limited, however. Also, the compliance issue can be vital in the adjuvant setting, and patient compliance was lower with this treatment than with adjuvant therapy in other tumour types.
Attempts to optimise results are based on the rationale that the analysis of expression of genes involved in DNA repair might be used to individualise optimal chemotherapy drug use. BRCA1 is known to have a role in the homologous recombination nucleotide excision repair pathway and to function as a differential regulator of response to cisplatin and anti-microtubule agents. BRCA1 levels can serve as both prognostic and potentially predictive factors. Low levels signify low risk and cisplatin sensitivity, while high levels are indicative of high risk and cisplatin resistance.
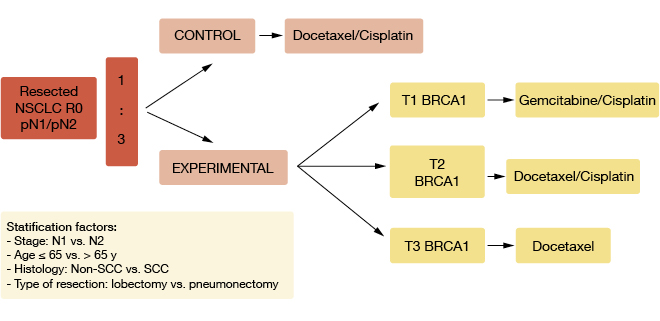
In the randomised, phase III, adjuvant SCAT trial, treatment was customised to BRCA1 status [3]. Patients with resected NSCLC and node involvement (R0 pN1/pN2) were randomly assigned to either docetaxel plus cisplatin (n = 108) or experimental therapy that was administered according to the BRCA1 levels (n = 392; Figure). Patients with low levels received gemcitabine/cisplatin; patients with intermediate levels were treated with docetaxel/cisplatin, and those with high levels received single-agent docetaxel. Post-operative radiotherapy was applied in patients with N2 disease. The primary endpoint was OS.
Figure: Design of the SCAT trial
Differential effects according to BRCA1 levels
BRCA1 expression levels were higher in tumours with squamous histology than in those with adenocarcinoma histology. For OS, a trend favoured experimental therapy over control therapy (HR, 0.86). In the subgroup with high BRCA1 levels, DFS and OS were inferior in the experimental arm that had received no cisplatin. Opposite results were noted in the group of patients with low BRCA1 levels: Here, the outcomes were better for those who had been treated with cisplatin/gemcitabine in the experimental arm than for patients receiving cisplatin/docetaxel in the control arm, with a HR for OS of 0.50. With regard to histology, no differences between the groups were seen for OS in squamous carcinoma, while in the adenocarcinoma group, there was a clear trend that indicated benefit in the experimental arm (HR, 0.66).
The full dose of planned treatment conferred a survival advantage (HR, 0.63; p = 0.04). Patients treated in the experimental arm required fewer dose reductions. No differential effects of the tested treatments were observed according to lymph node status.
Overall, this customisation of adjuvant chemotherapy according to BRCA1 levels was demonstrated to be feasible in node-positive resected NSCLC, although it did not significantly improve OS. The authors emphasised that longer follow-up is needed to confirm these data, as the median survival has not been reached yet.
REFERENCES
- Choy H et al., Concurrent pemetrexed and radiation therapy in the treatment of patients with inoperable stage III non-small cell lung cancer: a systematic review of completed and ongoing studies. Lung Cancer 2015; 87(3): 232-40
- Senan S et al., Final overall survival (OS) results of the phase III PROCLAIM trial: Pemetrexed (Pem), cisplatin (Cis) or etoposide (Eto), Cis plus thoracic radiation therapy (TRT) followed by consolidation cytotoxic chemotherapy (CTX) in locally advanced nonsquamous non-small cell lung cancer (nsNSCLC). Clin Oncol 33, 2015 (suppl; abstr 7506)
- Massuti B et al., Randomized phase III trial of customized adjuvant chemotherapy (CT) according BRCA-1 expression levels in patients with node positive resected non-small cell lung cancer (NSCLS) SCAT: A Spanish Lung Cancer Group trial (Eudract:2007-000067-15; NCTgov: 00478699). J Clin Oncol 33, 2015 (suppl; abstr 7507)
For more articles on cancer, lung cancer and respiratory medicine see
www.springermedizin.at/fachbereiche-a-z/i-o/innere-medizin/onkologie
www.springermedizin.at/fachbereiche-a-z/i-o/innere-medizin/pulmologie
http://bit.ly/1gFw0FJ
and www.inoncology.com
Dr. Judith Moser