New approaches are raising hope for SCLC patients
Only minor progress has been made over the past 30 to 40 years in the treatment of small-cell lung cancer (SCLC), which accounts for 10 % to 15 % of lung cancer cases. SCLC is radiosensitive, but approximately 70 % of patients present with extended disease that cannot be included within one radiotherapy field. The majority of patients respond to first-line chemotherapy. However, these responses are almost always transient, and outcomes with second-line treatments are generally poor. In extensive disease, median survival from the time of diagnosis does not usually exceed 10 months.
The current treatment paradigm for extensive-stage SCLC is combination chemotherapy in the first line (platinum–etoposide), followed by chemotherapy with topotecan, irinotecan, paclitaxel, docetaxel, and a variety of other drug choices for the recurrent/ progressive setting. All of these drugs elicit meagre responses. To date, topotecan is the only FDA-approved drug for recurrent disease. Alternatively, patients are enrolled into clinical trials, or receive only supportive care. No biomarker-driven therapies have been defined for this patient population yet.
There is an unmet need for the development of new active therapeutic options, particularly in relapsed disease. Meanwhile, however, improved understanding of SCLC biology has led to the identification of druggable targets; for the first time in decades, appropriate agents might start to change the course of the disease.
CheckMate 032
From the point of view of immunotherapy, SCLC is a ‘cold’ tumour, because the number of tumour-infiltrating T cells is low. This basically limits the efficacy of PD-1 antibodies, such as nivolumab, but it can be overcome by use of a combination strategy. The anti- CTLA-4 antibody ipilimumab increases the number of tumour-reactive T cells, thus enhancing the activity of nivolumab.
The 3-arm CheckMate 032 trial enrolled 216 patients with SCLC and progressive disease after at least one prior line of therapy, including a first-line platinum-based regimen [1]. Patients were not selected on the basis of PD-L1 expression. They were randomised to either nivolumab 3 mg/kg i. v. every 2 weeks as monotherapy (n = 98), nivolumab 1 mg/ kg plus ipilimumab 3 mg/kg i.v. every 3 weeks for 4 cycles (n = 61), or nivolumab 3 mg/kg plus ipilimumab 1 mg/kg i.v. every 3 weeks for 4 cycles (n = 54). Thereafter, single-agent nivolumab 3 mg/kg every 2 weeks was administered as maintenance therapy.
Best results with nivolumab plus ipilimumab
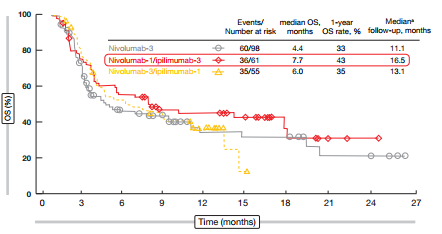
Nivolumab plus ipilimumab demonstrated greater efficacy than nivolumab alone. The ORR was 10 % with single-agent nivolumab, and twice as high with nivolumab-1 plus ipilimumab-3 (23 %) and nivolumab-3 plus ipilimumab-1 (19 %). As for patients with NSCLC, the responses were rapid and durable and occurred even in patients with bulky disease. They depended neither on platinum sensitivity nor on PD-L1 expression. For OS, the nivolumab-1/ipilimumab-3 combination appeared to have the most potent effects, with a median OS of 7.7 months (vs. 6.0 months for nivolumab-3/ipilimumab-1, and 4.4 months for nivolumab alone; Figure 1). Although a longer follow-up period is required, the analysis already indicates that a certain percentage of patients appears to experience long-term survival, which is a known effect of immunotherapeutic agents.
Figure 1: Superiority of nivolumab-1/ipilimumab-3 for overall survival in patients with recurrent SCLC, as compared to nivolumab-3/ipilimumab-1 and nivolumab monotherapy
The safety profiles here were similar to those seen with nivolumab and ipilimumab in other diseases. Higher rates of AEs occurred with combination therapy; there were also three treatment-related deaths (pneumonitis, myasthenia gravis, worsening of renal failure). Immune-related AEs were managed using established safety guidelines. However, only 10 % of patients discontinued treatment because of toxicity.
Nivolumab 1mg/kg plus ipilimumab 3 mg/kg was selected for the phase III investigations. Three studies are expected to confirm and extend these data. Apart from the CheckMate 032 expansion study, which is currently ongoing, Check-Mate 331 will be comparing nivolumab and chemotherapy in patients with relapsed SCLC, and CheckMate 451 will test nivolumab alone versus nivolumab plus ipilimumab versus placebo as consolidation/ maintenance therapy after platinum-based first-line treatment in patients with extensive disease.
Rova-T: the first biomarker directed strategy in SCLC
Delta-like protein 3 (DLL3) has been established as a novel target in neuroendocrine tumours. It is an atypical inhibitory Notch ligand, which is induced by the key neuroendocrine transcription factor, ASCL1. In SCLC, DLL3 is highly up-regulated and overexpressed; there is cell surface expression on both cancer stem cells and tumour cells (but not in normal adult tissue), which makes it amenable to an antibody–drug conjugate (ADC) approach. DLL3 is not a prognostic marker and does not predict response to cytotoxic chemotherapy.
Rovalpituzumab tesirine (Rova-TTM, SC16LD6.5) was designed as a DLL3-targeted ADC. Within this molecule, an anti-DLL3 monoclonal antibody is linked to the pyrrolobenzodiazepine (PBD) dimer toxin, which causes DNA strand breaks and is highly cytotoxic in a cell-cycle-independent manner. Due to selective binding of the antibody, the toxin is released within the cancer cells only.
The first-in-human, phase I, dose-escalation SCRX16-001 trial included 74 patients with SCLC [2]. Two expansion cohorts received 0.2 mg/kg every 3 weeks or 0.3 mg/kg every 6 weeks. Finally, the trial defined 0.3 mg/kg twice every 6 weeks as the recommended phase II dose. DLL3 expression was assessed using immunohistochemistry and was graded as low (≥ 1 % of tumour cells, 88 % of patients) or high (≥ 50 % of tumour cells, 67 % of patients).
Benefit irrespective of the number of prior lines
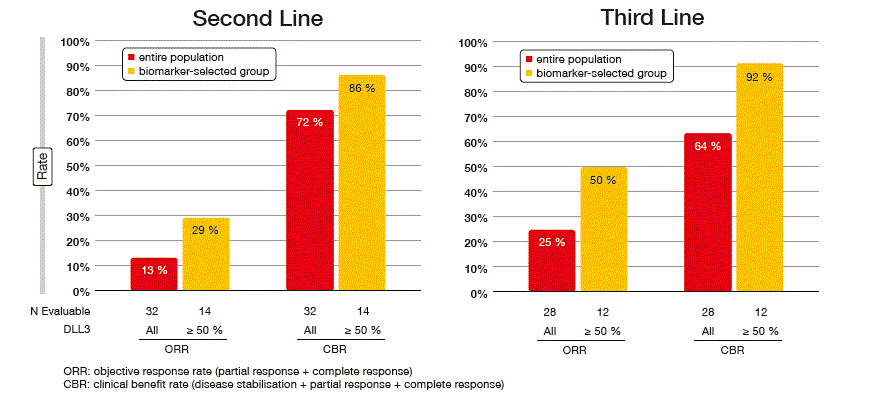
The SCRX16-001 trial showed substantial clinical activity of Rova-T, with DLL3 expression being predictive of response. RECIST-confirmed response rates per investigator were 18 % in the entire population and 39 % in the biomarker-selected group; the latter comprised patients with DLL3 expression of ≥ 50 %. Clinical benefit rates were 68 % and 89 %, respectively. In a subset of patients, a central review was performed that confirmed the per-investigator responses. The analysis revealed substantial benefit irrespective of the number of prior lines, with response rates comparable in the second-line and third-line settings (Figure 2). According to the waterfall plot, all of the responses occurred in the group with high DLL3 expression. The population with high DLL3 expression showed a median OS of 5.8 months and a 1-year survival rate of 32 %. Overall, the safety profile proved manageable. Among the grade 3 or higher AEs, thrombocytopenia occurred in 12 % of patients, serosal effusions in 11 %, and skin reactions in 8 %.
Figure 2: Comparable confirmed responses with Rova-T in the second-line and third-line setting of recurrent/ refractory SCLC
Historical comparisons illustrate that second-line and third-line results gained with Rova-T are superior to standard-of-care therapy. However, the numbers of patients involved in the SCRX16-001 trial are small; for the time being, these data are only hypothesis generating, but they are promising, and they justify further clinical development. The phase II, single-arm TRINITY trial is currently enrolling in the thirdline setting, and this will be the confirmatory trial for SCRX16-001. Additional studies, such as a first-line basket trial investigating the activity of Rova-T in other tumour types that express DLL3, are in the planning phases.
PARP inhibition in addition to chemotherapy
A multi-centre, randomised, doubleblind, phase II study tested the oral PARP-1/2 inhibitor veliparib at a dose of 40 mg twice daily for 7 days in addition to temozolomide, in patients with relapsed sensitive and refractory SCLC after failure of one or two prior regimens [3]. Sensitive disease was defined as relapse 60 days after completion of firstline platinum-based chemotherapy, whereas refractory patients had shown no response to initial platinum-based therapy or had experienced progression within 60 days after completing treatment; furthermore, this definition included any patient in need of third-line therapy. Fifty-five patients received veliparib in addition to temozolomide, and 40 patients were treated with placebo plus temozolomide. Veliparib was chosen because SCLC has been characterised by aberrant expression of genes and proteins implicated in DNA damage repair. Therefore, DNA repair pathways represent an attractive target in SCLC.
PFS and OS did not differ significantly between the veliparib and placebo arms, but there was a significant difference regarding the ORR (39 % vs.14 %; p = 0.016). Expression of proteins involved in DNA repair, such as PARP-1, SLFN11 and MGMT, was assessed and correlated with outcome. Here, a trend towards better OS was observed with high SLFN11 expression in the veliparib arm. The analysis for additional biomarkers is ongoing. Moreover, these findings indicate that increased numbers of circulating tumour cells at baseline and after cycle 1 are associated with poorer survival. The improved ORR observed in this study supports further trials of PARP1 inhibitors and temozolomide in SCLC.
Anti-angiogenesis with bevacizumab and pazopanib
As angiogenesis is abundant in SCLC and associated with poor prognosis, another promising approach consists of inhibition of VEGF using the anti-VEGF antibody bevacizumab. In a multi-centre, phase III study, the addition of bevacizumab to platinum and etoposide in the first-line treatment of extensivestage SCLC gave rise to a significant improvement in PFS, compared to the control regimen, which consisted of cisplatin and etoposide only (1-year PFS rates, 18.4 % vs. 11.5 %; HR, 0.72) [4]. Approximately 100 patients were treated in each arm. For OS, which was defined as the primary outcome, the analysis demonstrated a non-significant advantage of the bevacizumab-based therapy (1-year OS rate, 36.7 % vs. 24.9 %; HR, 0.78). Response rates did not differ significantly between the two groups. Time-dependent analysis revealed a significant effect of the maintenance treatment on OS (HR, 0.60). The toxicity profile was acceptable. As the authors noted, further research in the area of anti-angiogenetic treatments of SCLC is warranted.
Anti-angiogenic effects are also elicited by the multikinase inhibitor pazopanib, which is directed against VEGFR, PDGFR, FGFR and c-KIT. The Hellenic Oncology Research Group conducted a non-randomised, open-label, phase II trial on single-agent pazopanib at a dose of 800 mg/day as second-line treatment in patients with both chemoresistant/ chemorefractory and chemosensitive SCLC [5]. Patients with sensitive relapse were included in Cohort A (n = 39), and those with resistant or refractory disease were included in Cohort B (n = 19). The primary objective was the progressionfree rate (PFR) at week 8.
This trial met its primary endpoint for Cohort A; here, PFR was 59 %. Recruitment in Cohort B was terminated early due to lack of efficacy at the interim analysis (PFR, 26.3 %). In Cohort A, median PFS and median OS were 3.7 and 8.0 months, respectively. At 1 year, 26.5 % of these patients were alive. Pazopanib was well tolerated.
This was the first study to demonstrate substantial and clinically relevant efficacy of a tyrosine kinase anti-angiogenic inhibitor as a salvage treatment in patients with SCLC. The authors concluded that pazopanib should be evaluated further as monotherapy or in combination with other agents.
CONVERT: chemoradiotherapy in limited disease
Approximately one third of patients with SCLC present with limited-stage disease. In those with good performance status, the standard of care is concurrent chemoradiotherapy (CTRT). The best outcomes have been documented for twice-daily (BD) CTRT; however, only one fifth of the patients actually use BD treatment routinely, due to toxicity and logistic issues. There has been a lack of consensus on the standard radiotherapy regimens in limited- stage SCLC, which led to the development of the multinational, phase III CONVERT trial [6].
Here, patients were randomly assigned to receive either 45 Gy in 30 fractions BD for 3 weeks (n = 274), or 66 Gy in 33 fractions once daily (OD) for 6.5 weeks (n = 273). Chemotherapy consisted of 4 to 6 cycles of cisplatin and etoposide. Radiotherapy started on day 22 of cycle 1. OS was defined as the primary endpoint of the CONVERT trial.
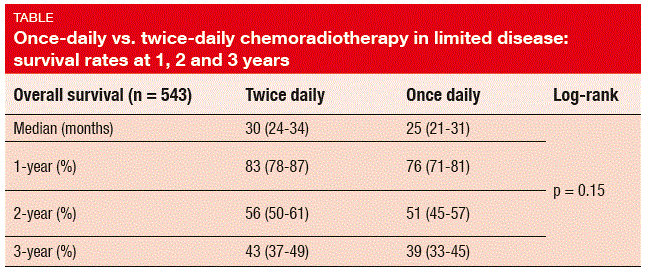
Even though the radiotherapy treatment delivery was higher in the BD arm, OS was comparable across these two groups, with 2-year survival rates of 56 % and 51 % for the BD and OD arms, respectively (HR 1.17; p = 0.15; Table). OD radiotherapy did not result in worse toxicity than BD radiotherapy. Toxicities were comparable, except for significantly higher rates of grade 3/4 neutropenia with BD treatment. Grade 3/4 acute oesophagitis occurred in 19 % in both arms, and grade 3/4 acute radiation pneumonitis was generally rare (2.5 % and 2.2 % with BD and OD treatments, respectively). Overall, radiation-related toxicity arose less frequently than expected, which is probably due to the use of modern radiotherapy techniques. The results of CONVERT support the use of either regimen as the standard-of-care treatment of limited-stage SCLC patients with good performance status.
REFERENCES
- Antonia SJ et al., CheckMate 032: nivolumab alone or in combination with ipilimumab for the treatment of recurrent small cell lung cancer. J Clin Oncol 34, 2016 (suppl; abstr 100)
- Rudin CM et al., Safety and efficacy of single agent rovalpituzumab tesirine (SC16LD6.5), a deltalike protein 3 (DLL3)-targeted antibody-drug conjugate (ADC) in recurrent or refractory small cell lung cancer (SCLC). J Clin Oncol 34, 2016 (suppl; abstr LBA8505)
- Pietanza MC et al., A multi-center, randomized, double-blind phase II study comparing temozolomide plus veliparib, a PARP-inhibitor, or placebo as 2nd or 3rd-line therapy for patients with relapsed small cell lung cancers. J Clin Oncol 34, 2016 (suppl; abstr 8512)
- Tiseo M et al., Italian multicentre phase III randomised study of cisplatin-etoposide with or without bevacizumab as first-line treatment in extensive small cell lung cancer (SCLC): GOIRC-AIFA FARM- 6PMF JM trial. J Clin Oncol 34, 2016 (suppl; abstr 8513)
- Kotsakis A et al., Salvage treatment of relapsed/ refractory small cell lung cancer with pazopanib: a Hellenic Oncology Research Group’s (HORG) phase II study. J Clin Oncol 34, 2016 (suppl; abstr 8561)
- Faivre-Finn C et al., Concurrent ONce-daily Versus twice-daily RadioTherapy: a 2-arm randomised controlled trial of concurrent chemo-radiotherapy comparing twice-daily and once-daily radiotherapy schedules in patients with limited-stage small cell lung cancer and good performance status. J Clin Oncol 34, 2016 (suppl; abstr 8504)