Recent benchmarks in the management of small-cell tumours
KEYNOTE-158
Extensive-disease small-cell lung cancer (ED-SCLC) is highly responsive to first-line therapy, but early relapses commonly occur, and prognosis is poor. To date, no biomarker-driven therapies have been established.
Based on the involvement of the immune system in the pathophysiology of SCLC and the high mutational burden of this disease, immunotherapy has potential as a novel treatment option [1-3]. KEYNOTE-158, a phase II basket study conducted in 10 tumour types including cancer with high microsatellite instability (MSI-H), assessed the anti-PD1 antibody pembrolizumab in advanced SCLC regardless of biomarker status [4]. Pre-treated patients with unresectable and/or metastatic SCLC who had experienced progression on or intolerance to standard therapy received pembrolizumab 200 mg Q3W for 2 years or until progression. ORR constituted the primary endpoint. Out of 107 patients, 16 (15 %) had stable brain metastases. The cohort included one patient with carcinoid histology and seven patients with neuroendocrine tumours.
One third of the population had already received 2 treatment lines, and in 23 %, ≥ 3 therapies had been administered. At baseline, 39 % and 47 % of tumours were PD-L1–positive and PD-L1–negative, respectively, with 14 % being not evaluable. Furthermore, the biomarker analysis yielded non–MSI-H status (i.e., microsatellite stability and low microsatellite instability) in 91 %, with 9 % being not evaluable.
Superior results in the PD-L1–positive group
Overall, responses occurred in 18.7 %, and disease control was observed in 30 %. Patients with PD-L1–positive tumours responded in 35.7 %, while in the PD-L1–negative group, this was only the case in 6.0 %. Disease control rates amounted to 43 % and 20 %, respectively. The authors pointed out that these findings are consistent with those from the SCLC cohort of the phase IB KEYNOTE-028 trial that evaluated pembrolizumab in patients with previously treated extensive-stage tumours expressing PD-L1 [5]. Importantly, responses proved durable; their median duration had not been reached at the time of the analysis, and 12 patients (73 %) responded for ≥ 1 year. Median PFS was 2.1 and 1.9 months for PD-L1–positive and PD-L1–negative patients, respectively. The respective OS results were 14.9 and 5.9 months (Figure 1). At 12 months, 53.1 % and 30.7 % of patients, respectively, were alive.
The safety profile matched the previous experience for pembrolizumab monotherapy in other tumour types. Pembrolizumab plus standard-of-care chemotherapy (i.e., etoposide/platinum) is being evaluated in the ongoing phase III KEYNOTE-604 study in patients with newly diagnosed ED-SCLC.
Figure 1: Promising survival results in the PD-L1–positive patient group treated with pembrolizumab
Durvalumab alone and together with tremelimumab
Another checkpoint inhibitor investigated in SCLC is the anti-PD-L1 antibody durvalumab. This agent showed activity both as a single agent and in combination with the anti-CTLA-4 antibody tremelimumab. In the monotherapy trial, which was the multicentre, open-label ED-SCLC expansion cohort of Study 1108, durvalumab 10 mg/kg Q2W for up to 12 months demonstrated durable clinical activity in certain patients [6]. Only 2 out of 21 patients responded (ORR, 9.5 %), but these responses lasted 14.6 and 29.5+ months, respectively. The second patient was platinum-refractory and had received 3 prior treatment lines. Median PFS and OS were 1.5 and 4.8 months, respectively. The 12-month OS rate amounted to 27.6 %. Durvalumab was well tolerated, with no grade-3/4 AEs observed.
In the multicentre, open-label combination trial, which was a phase I dose exploration/expansion study, durvalumab 20 mg/kg Q4W and tremelimumab 1 mg/kg Q4W for 4 doses were tested in previously treated patients with select advanced solid tumours. After the combination phase, patients received durvalumab 10 mg/kg Q2W to complete 12 months. At the ASCO 2018 Congress, Cho et al. presented the first report of clinical activity and safety in the ED-SCLC dose-expansion cohort (n = 30) [7].
Consistent with findings in NSCLC [8], durvalumab plus tremelimumab demonstrated promising activity. Confirmed ORR was 13.3 %, including 2 complete and 2 partial responses. Three platinum-resistant/-refractory patients responded to the treatment. Responses occurred early on and were durable (median DOR, 18.9 months). The 6-month PFS rate was 16.3 %, and the 12-month OS rate was 41.7 %. There were no discontinuations or deaths due to treatment-related AEs. Grade-3/4 treatment-related AEs occurred in 23.3 %. The authors concluded that together with the monotherapy data, these results indicate activity of durvalumab in patients with ED-SCLC. Ongoing trials include the phase II, open-label BALTIC study investigating durvalumab plus tremelimumab in platinum-refractory patients and the phase III CASPIAN trial into first-line durvalumab with or without tremelimumab plus platinum-based chemotherapy versus chemotherapy alone.
Compelling activity of second-line lurbectedin monotherapy
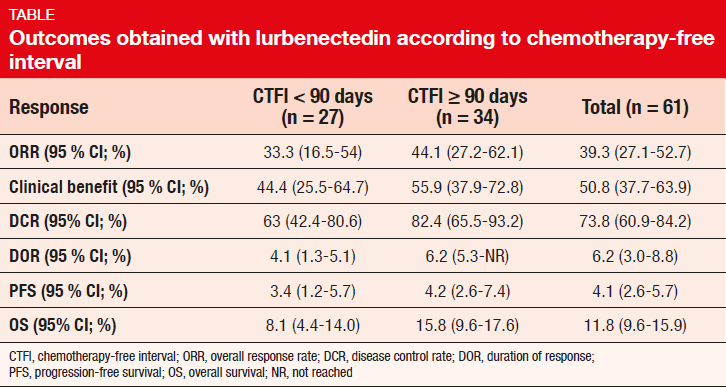
Lurbinectedin is a new anti-cancer drug that blocks transcription and induces DNA double-strand breaks, leading to apoptosis. Trigo et al. presented the results of 61 SCLC patients treated in a multicentre phase II basket trial that is assessing the efficacy and safety of lurbinectedin 3.2 mg/m2 Q3W in several types of advanced solid tumours [9]. The SCLC group had received one prior chemotherapy line. Brain metastases were not allowed. For this analysis, the population was split up according to the chemotherapy-free interval (CTFI); 34 and 27 patients had a CTFI of ≥ 90 and < 90 days, respectively.
The group with prolonged CTFI was shown to fare better with respect to ORR, clinical benefit, disease control rate, DOR, PFS, and OS (Table). At 6 months, 36.3 % of patients in the overall population were alive and progression-free; these proportions were 42.8 % and 28.1 % for CTFI ≥ 90 and < 90 days, respectively. Likewise, OS rates at 12 months were 59.1 % and 22.9 %. The safety profile observed in this population was acceptable and well tolerated, with no unexpected toxicity or drug-related deaths. According to the investigators, these results suggest that single-agent lurbinectedin can be considered as an alternative therapy for patients with relapsed SCLC.
Rova-T in the third-line setting
A novel target in neuroendocrine tumours is the atypical inhibitory Notch ligand delta-like protein 3 (DLL 3). It is expressed on both cancer stem cells and tumour cells, but not on normal adult tissues. More than 85 % of SCLC express DLL3, although it is not prognostic of outcomes on standard therapy. The antibody-drug conjugate rovalpituzumab tesirine (Rova-TTM) has been developed to target DLL3. A phase I study demonstrated an ORR of 16 % in 56 patients with recurrent SCLC; here, those with the highest DLL3 expression responded in 31 % and experienced a median OS of 5.8 months [10].
The phase II, single-arm TRINITY trial tested Rova-T 0.3 mg/kg (2 doses, 6 weeks apart) in 339 patients with DLL3-expressing, relapsed or refractory SCLC who had already been treated with ≥ 2 previous regimens containing at least 1 platinum-based regimen [11]. Re-treatment was permitted at progression. Seventy percent of the patients participating in TRINITY were defined as DLL3-high, i.e. ≥ 75 % of cells in their tumours expressed DLL3. Stable CNS metastases were allowed. Among the 339 patients enrolled, 23 % were resistant or refractory to first-line platinum therapy. Seventy-seven percent had been pre-treated with 2 lines. Brain metastases were present in 40 %, and 25 % had a history of pleural effusions.
Clinical benefits in > 70 %
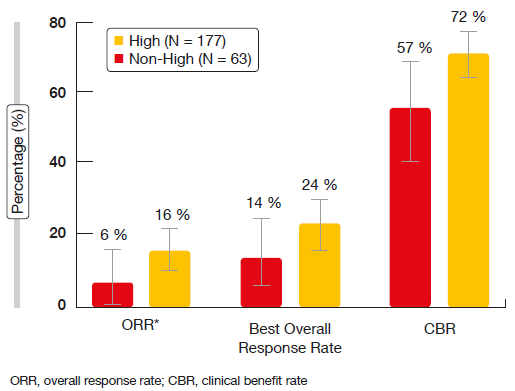
ORR constituted the primary endpoint. According to the investigators, ORR was 18.0 % in the entire cohort and 19.7 % in the DLL3-high subgroup. These results were 12.4 % and 14.3 % per IRC. Median OS amounted to 5.6 and 5.7 months for the overall group and the DLL3-high patients, respectively. Response rates appeared to be higher in the third- and fourth-line settings (29 % and 23 % for third and fourth line, respectively, according to investigator). Importantly, clinical benefit rates (complete and partial responses plus disease stabilisations) were > 70 % in the third and fourth line according to both investigator and IRC. The responses seemed to be enriched in the group with high DLL3 expression. There was a tendency to improvement in ORR, best overall response rate and clinical benefit rate in the DLL3-high patients as opposed to those with non-high, but positive DLL3 expression (Figure 2). Approximately 40 % of responses occurred after 10 weeks of treatment initiation. DOR by IRC was 4.1 and 2.8 months in the third- and fourth-line setting, respectively. IRC-assessed PFS and OS among DLL3-high patients in all lines were 3.8 and 5.7 months, respectively.
Drug-related serious AEs occurred in 30 %, and grade ≥ 3 AEs in 40 %. Ten fatal AEs (3 %) occurred during the study due to generalised oedema, pneumonitis, ascites, drug-induced liver injury, pleural effusion, pneumothorax, respiratory failure, and sepsis. In 5 %, AEs led to discontinuation. The most common AEs included photosensitivity reactions (35 %), pleural effusions (28 %), fatigue (28 %), peripheral oedema (26 %), and thrombocytopenia (22 %). The risk of high-grade serosal effusions appeared to be increased in patients who had already developed effusions before treatment.
The investigators concluded that Rova-T is an active agent in SCLC beyond the second treatment line, where currently no therapies are approved. Rova-T is being evaluated in the MERU and TAHOE phase III studies that are assessing this drug in frontline maintenance and in the second-line setting, respectively. Multiple phase I trials are also ongoing; these are testing Rova-T in combination with chemotherapy, nivolumab, and nivolumab/ipilimumab.
Figure 2: ORR, best overall responses and clinical benefits in DLL3-high and DLL3–non-high patients
What happens after SCLC transformation?
Three to 10 % of EGFR-mutant adenocarcinomas transform to high-grade neuroendocrine carcinoma, including SCLC, at acquired resistance to TKI treatment [12]. Cases of de novo SCLC harbouring an EGFR mutation have been reported [13]. As characteristics and clinical course of SCLC-transformed EGFR-positive lung cancer were largely unknown, Marcoux et al. retrospectively reviewed 67 patients with EGFR-mutant SCLC [14]. At initial diagnosis of metastatic cancer, 58 (87 %) had pathology consistent with NSCLC, and 9 (13 %) had evidence of SCLC. Five had pure SCLC, and 4 had mixed histology that included a SCLC component. The patients received a median of 2 systemic treatment lines before transformation. At the time of transformation, 93 % were treated with an EGFR TKI. Median time between the initial diagnosis of metastatic NSCLC and the first evidence of SCLC was 17.8 months.
All genotyped patients kept their founder EGFR mutation at transformation. The majority of previously T790M-positive patients (79 %) no longer had T790M detected at the time of transformation. TP53, RB1 and PIK3CA mutations were the next most frequently observed genetic alterations. Median OS from initial diagnosis of metastatic lung cancer (31.5 months) was similar to the expected OS in EGFR-mutant patients that never transform to SCLC, and median OS from first evidence of EGFR-mutant SCLC (10.9 months) was similar to what is seen with de novo SCLC. Responses to platinum-etoposide and taxanes were frequent, but transient. Importantly, the analysis revealed no responses to checkpoint inhibitor therapy. The authors summarised that further investigation is required to better elucidate the optimal diagnostic and therapeutic approach for these EGFR-mutant tumours.
REFERENCES
- George J et al., Comprehensive genomic profiles of small cell lung cancer. Nature 2015; 524(7563): 47-53
- Maddison P et al., Favourable prognosis in Lambert-Eaton myasthenic syndrome and small-cell lung carcinoma. Lancet 1999;
353(9147): 117-118 - Peifer M et al., Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012; 44: 1104-1110
- Chung HC et al., Phase 2 study of pembrolizumab in advanced small-cell lung cancer: KEYNOTE-158. J Clin Oncol 36, 2018 (suppl; abstr 8506)
- Ott PA et al., Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol 2017; 35(34): 3823-3829
- Goldman JW et al., Safety and antitumor activity of durvalumab monotherapy in patients with pretreated extensive disease small-cell lung cancer. J Clin Oncol 36, 2018 (suppl; abstr 8518)
- Cho D et al., Safety and clinical activity of durvalumab in combination with tremelimumab in extensive disease small-cell lung cancer. J Clin Oncol 36, 2018 (suppl; abstr 8517)
- Antonia S et al., Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016; 17; 299-308
- Trigo JM et al., Efficacy and safety of lurbinectedin (PM1183, Zepsyre®) in small cell lung cancer (SCLC): results from a phase 2 study. J Clin Oncol 36, 2018 (suppl; abstr 8570)
- Rudin CM et al., Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017; 18(1): 42-51
- Carbone DP et al., Efficacy and safety of rovalpituzumab tesirine (Rova-TTM) in patients with DLL3-expressing, ≥ 3rd line small cell lung cancer: results from the phase 2 TRINITY study. J Clin Oncol 36, 2018 (suppl; abstr 8507)
- Sequist LV et al., Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3(75): 75ra26
- Okamoto I et al., EGFR mutation in gefitinib-responsive small-cell lung cancer. Ann Oncol 2006; 17(6): 1028-1029
- Marcoux N et al., Outcomes of EGFR-mutant lung adenocarcinomas that transform to small cell lung cancer. J Clin Oncol 36, 2018
(suppl; abstr 8573)