Novel first-line options and other insights in EGFR-mutant lung cancer
RELAY: addition of ramucirumab
Although treatment with EGFR TKIs is generally efficient in patients with EGFR-mutant lung cancer, resistance inevitably develops within 8 to 12 months of the initiation of therapy, leading to treatment failure. Therefore, there is an unmet need for options that extend the activity of EGFR-targeted therapies. Dual blockade of the VEGF and EGFR signaling pathways represents a potential approach in this respect.
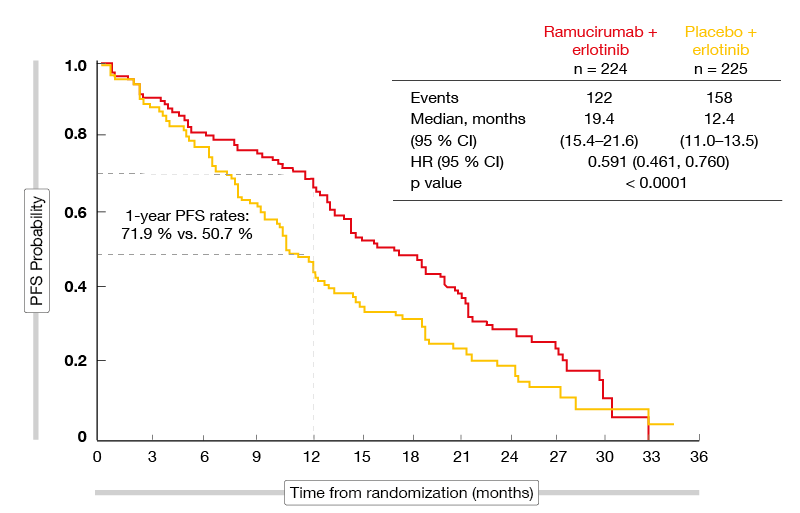
The global, randomized, placebo-controlled phase III RELAY trial tested the combination of the first-generation EGFR TKI erlotinib with the anti-VEGFR-2 antibody ramucirumab as first-line strategy in patients with stage IV, EGFR-mutation–positive (i.e., exon 19 deletion, exon 21 L858R mutation) NSCLC [1]. Patients with brain metastases were excluded. The treatment consisted of either erlotinib 150 mg/d plus ramucirumab 10 mg/kg every 2 weeks (n = 224) or erlotinib 150 mg/d plus placebo (n = 225). Treatment was continued until progression or unacceptable toxicity. Investigator-assessed PFS constituted the primary endpoint. Hundred centers in 13 countries participated in the study. In both arms, 77 % of patients were Asian.
The addition of ramucirumab led to a significant 7-month PFS improvement that translated into a highly statistically significant risk reduction of 41 % (19.4 vs. 12.4 months; HR, 0.591; p < 0.0001; Figure). The Kaplan Meier curves separated from the beginning. At 1 year, 71.9 % vs. 50.7 % of patients were progression-free. Independent blinded central review showed a consistent PFS benefit (HR, 0.671; p = 0.0022). Ramucirumab plus erlotinib gave rise to PFS benefits across most of the subgroups. Notably, patients with exon 19 deletions and exon 21 L858R mutations derived similar benefits (HRs, 0.651 and 0.618, respectively).
Figure: Seven-month PFS improvement with the addition of ramucirumab to erlotinib in the RELAY trial
Secondary endpoints
Overall response rates were comparable across the two arms (76 % vs. 75 %), which also applied to disease control rates (95 % vs. 96 %). However, duration of response was significantly longer with the ramucirumab-based regimen (18.0 vs. 11.1 months; HR, 0.619). OS outcomes are still immature. For PFS2, i.e. the time from randomization to second disease progression, the analysis revealed a significant advantage for the combination (HR, 0.690; p = 0.03). This implies freedom from progression as well as an OS benefit. The incidence of the EGFR T790M resistance mutation was assessed using liquid biopsy at baseline and one month later. While no T790M mutations were detected at baseline in either group, mutation rates at 30 days in patients experiencing progression were similar at 43 % vs. 47 %.
The safety results were consistent with the established profiles for erlotinib and ramucirumab. Grade ≥ 3 treatment-emergent AEs occurred more frequently in the combination arm (72 % vs. 54 %). However, discontinuation rates for all study treatments due to AEs did not differ (13 % vs. 11 %). Any-grade and severe hypertension was more prevalent in the experimental arm, although there were no cases of grade 4 hypertension. Similarly, ramucirumab-treated patients more frequently developed elevations of transaminases, but most events were rated as grade 1 or 2. This also applied to bleeding events. Overall, these findings suggest that erlotinib plus ramucirumab is a new option for the initial treatment of patients with EGFR-mutant metastatic NSCLC.
Gefitinib plus chemotherapy
Another strategy to delay or prevent acquired resistance to first-line EGFR TKI therapy is the combination of oral TKIs with chemotherapy. At the ASCO 2019 Congress, Noronha et al. presented the results of a randomized, open-label phase III trial comparing the first-generation EGFR TKI gefitinib with gefitinib plus pemetrexed/carboplatin for four cycles followed by pemetrexed maintenance in patients with non-progressive disease [2]. Patients with stage IIIB or IV, EGFR-mutant NSCLC received either the combination (n = 174) or the gefitinib monotherapy (n = 176) with first-line palliative intent until progression. More than 20 % of patients in each arm had ECOG PS 2. In 17 % and 19 %, respectively, brain metastases were present. Patients with rare EGFR aberrations, such as exon 18 and exon 20 mutations, were included.
The combined approach induced significant improvements compared to gefitinib alone regarding both PFS and OS. Median PFS, which was defined as the primary endpoint, was doubled (16 vs. 8 months; HR, 0.51; p < 0.0001). Significant PFS benefits occurred across all of the subgroups. Concerning OS, the mortality risk was reduced by 55 % (not reached vs. 17 months; HR, 0.45; p < 0.0001). The patients in the combined arm also showed more pronounced responses (ORR, 75.3 % vs. 62.5 %; p = 0.01) and median depth of response (-56.4 vs. -43.5; p = 0.002). Clinically relevant grade ≥ 3 toxicities doubled from 25.3 % to 50.6 % (p < 0.001). Most of these were cytopenias. Except for nephrotoxicity and hypokalemia, there were no significant increases across the two arms for other types of toxicity.
These results establish gefitinib plus pemetrexed/carboplatin as an additional first-line option for patients with EGFR-mutant NSCLC. The investigators pointed out that this is one of the few regimens that prolongs OS in this setting. The PFS benefit resembled that obtained in the FLAURA trial conducted with the third-generation EGFR TKI osimertinib, although in the present study, patients with PS 2 were included, whereas FLAURA enrolled only patients with PS ≤ 1 [3]. According to the authors, sequencing of effective therapies is important to maximize survival. As osimertinib is active in T790M-mutation–positive tumors, it might be better positioned in the relapsed setting.
TAK-788 works for exon 20 insertions
Currently approved EGFR TKIs have shown efficacy in lung tumors with common activating EGFR mutations including exon 19 deletion and exon 21 L858R mutation but are largely ineffective in patients with EGFR exon 20 insertions. Targeted options for patients with these aberrations, which occur in approximately 6 % of cases, are lacking [4].
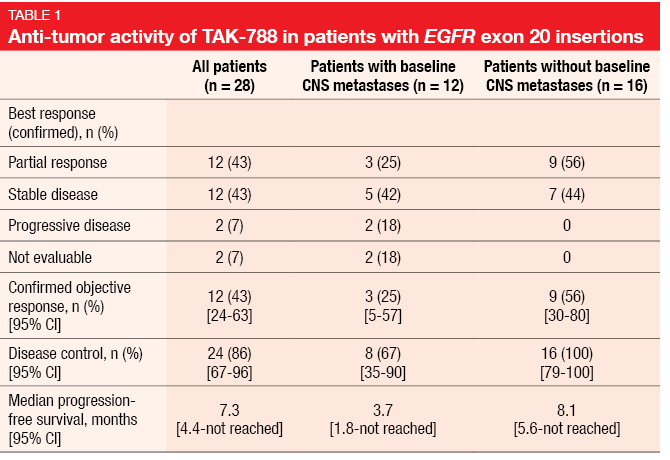
The experimental EGFR TKI TAK-788 was shown to potently inhibit exon 20 mutations with selectivity over wild-type EGFR. TAK-788 is being evaluated in a phase II trial testing 160 mg/d in 7 patient cohorts with NSCLC and other tumor types. At ASCO 2019, Jänne et al. reported the findings for cohort 1 that had refractory, exon-20–positive lung cancer and either active or measurable CNS metastases (but not both) [5]. One or more prior regimens of systemic therapy were required for inclusion; prior TKI therapy was allowed if there had been no response. The efficacy population comprised 28 patients. Median time on treatment was 7.9 months, and 50 % remained on study at the time of the analysis.
TAK-788 160 mg/d demonstrated anti-tumor activity, with a confirmed ORR of 43 % (Table 1). Patients with baseline CNS metastases responded in 25 %, while those without had a response rate of 56 %. In the total population, DCR and PFS amounted to 86 % and 7.3 months, respectively. Responses occurred in patients with various exon 20 insertion variants, including 769_ASV and 773_NPH. AEs proved manageable and consistent with those of other EGFR TKIs, with diarrhea, nausea, and rash being reported as the most common toxicities. Most of the treatment-related AEs were grade 1 and 2 and reversible. In the group of patients treated with at least one dose of TAK-788 160 mg/d during dose escalation or expansion in cohorts 1 to 7 (n = 72), treatment-related grade ≥ 3 AEs emerged in 40 %, and dose reductions became necessary in 25 %. In 14 %, treatment had to be discontinued due to toxicity.
The global EXCLAIM Extension Cohort is further investigating TAK-788 160 mg/d until progression in 91 patients with locally advanced or metastatic NSCLC harboring EGFR exon 20 insertions who have received 1 to 2 prior chemotherapies. Treated CNS metastases are allowed in this population. Confirmed ORR per independent review committee constitutes the primary endpoint.
Real-world experience with afatinib
Observational real-world data obtained in 88 Chinese patients confirmed the efficacy and tolerability of first-line treatment with the second-generation EGFR TKI afatinib [6]. ORR and DCR were 54.5 % and 92.0 %, respectively, and median PFS was 14.2 months. The activity of afatinib was not affected by the presence of brain metastases, dosage or treatment line. Among patients who progressed on afatinib, 65.4 % harbored the T790M mutation. Most of these received third-generation EGFR TKI treatment. Twenty-seven patients continued afatinib treatment beyond tumor progression; this strategy delayed the progression of disease symptoms. Median time to progression of clinical symptoms was 16.3 months.
A retrospective real-world study including 45 patients treated with first-line afatinib at several Spanish hospitals revealed an ORR of 68.9 %, with CR and PR rates of 13.3 % and 55.6 %, respectively [7]. Stable disease occurred in 17.8 %. Median PFS was 27 months, thus exceeding results obtained in clinical trials, and OS had not been reached yet. The authors surmised that the favorable PFS outcomes might be due to a large proportion of patients harboring tumors with exon 19 deletions that respond particularly well to afatinib.
The same cohort of patients was analyzed for the activity of afatinib in advanced age groups [8]. Median age was 71.2 years, with 24 patients (53.3 %) being ≥ 70 years old. Of course, compared to younger patients, the elderly required treatment interruptions and dose adjustments more frequently, but this did not appear to impair safety or efficacy. Afatinib doses were reduced in 47.6% of patients < 70 years and in 75 % of those aged ≥ 70. Treatment discontinuations due to AEs became necessary in 14.3 % vs. 20.8 %. ORRs obtained with afatinib were 76 % and 62.5 %, respectively, and disease control occurred in 90.3 % vs. 83.3 %. Median PFS was 20 months in the younger group but had not been reached in elderly patients yet.
Clinical implications of certain mutations
A Taiwanese analysis retrospectively evaluated the outcomes of 269 patients with EGFR-mutant NSCLC and brain metastases [9]. EGFR mutations were categorized into exon 19 deletions, L858R mutations, and uncommon mutations. The results suggested mutation-related differences regarding the natural history of disease and prognosis in patients with brain metastases, with uncommon mutations conferring poorer outcomes. Median PFS for patients with exon 19 deletions, L858R mutations and uncommon mutations was 10.4, 10.0, and 3.2 months, respectively (p = 0.03). For OS, this was 18.1, 17.4, and 12.5 months, respectively (p = 0.05). Compared to gefitinib, treatment with afatinib proved to be a favorable prognostic factor regarding both PFS (HR, 0.57; p = 0.03) and OS (HR, 0.48; p = 0.03).
Clinical features and progression patterns according to the presence of the EGFR T790M resistance mutation were the objective of an observational study conducted in Italy [10]. The cohort included 219 patients with EGFR-mutant NSCLC who progressed after first-line treatment with gefitinib, erlotinib, or afatinib. Forty-nine percent of patients acquired the T790M mutation. The emergence of T790M was shown to correlate with age < 65 years (p = 0.05) and the presence of exon 19 deletions (p = 0.04). This association was confirmed by a multivariate analysis (p = 0.010 and p = 0.006, respectively). At the time of progression, the T790M-positive group more commonly showed new progression sites (p = 0.005) as well as liver metastases (p < 0.001). The multivariate analysis confirmed the statistical significance of this observation (p = 0.01 and p = 0.008, respectively). Both univariate and multivariate analyses revealed longer median OS in T790M-positive patients (53 vs. 22 months according to univariate analysis; p < 0.0001).
Early ctDNA clearance: first-line osimertinib …
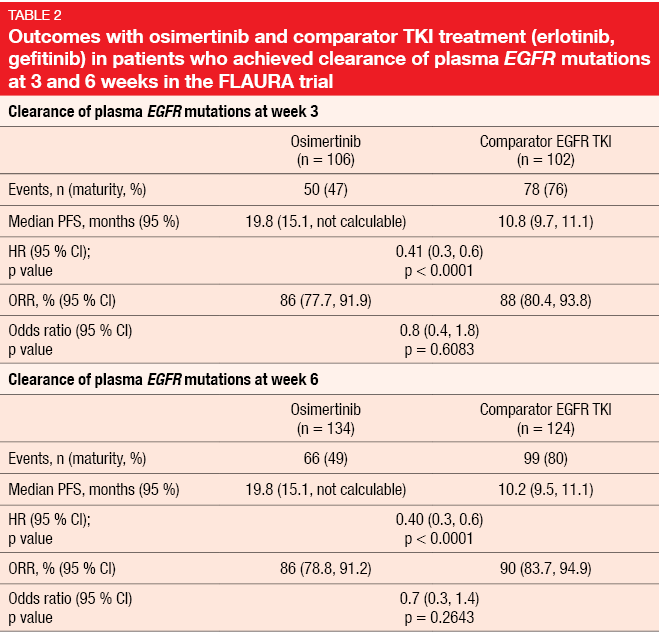
In the double-blind, randomized, phase III FLAURA trial, first-line treatment with osimertinib resulted in superior PFS compared to EGFR TKI therapy with erlotinib or gefitinib in patients with EGFR-positive, advanced NSCLC [3]. Early clearance of circulating tumor DNA (ctDNA) was found to correlate with PFS improvement in the AURA trials [11, 12]. Zhou et al. presented an exploratory analysis of the clinical outcomes associated with the detection of EGFR mutations at 3 or 6 weeks after the start of treatment in FLAURA to determine if early ctDNA clearance predicts PFS and ORR [13]. Evaluable ctDNA results at baseline and at weeks 3 and/or 6 were available for 244 and 245 patients in the osimertinib and comparator arms, respectively.
Indeed, the early clearance of plasma EGFR mutations appeared to be a prognostic factor for improved outcome. PFS was significantly prolonged in patients who showed clearance of their EGFR mutations compared to those who did not both at week 3 (13.5 vs. 9.5 months; HR, 0.57; p < 0.0001) and 6 (13.5 vs. 8.2 months; HR, 0.51; p < 0.0001). Within the group of patients who experienced EGFR mutation clearance, those receiving osimertinib showed significantly longer PFS both at week 3 (19.8 vs. 10.8 months; HR, 0.41; p < 0.0001) and 6 (19.8 vs. 10.2 months; HR, 0.40; p < 0.0001; Table 2). Likewise, if no clearance was achieved at 3 weeks, osimertinib-treated patients fared significantly better regarding PFS than those treated with first-generation EGFR TKIs (11.3 vs. 7.0 months; HR, 0.50; p = 0.001). At 6 weeks, the analysis revealed a trend in favor of osimertinib. Before the start of treatment, persisting EGFR mutations indicated worse outcomes, with median PFS of 11.1 versus 19.1 months in those who had obtained clearance. ORRs were generally similar across arms without any statistical significances, ranging from 73 % to 90 %.
Overall, these data suggest that patients at increased risk of rapid progression or death on first-line osimertinib treatment could be identified early on. A series analysis of additional timepoints over the course of treatment is underway. Further analyses are investigating the mechanism underlying the high risk of early progression in patients with detectable EGFR mutations following EGFR TKI therapy.
… and later-line osimertinib
Similarly, Song et al. showed that ctDNA clearance within 50 days of the initiation of osimertinib treatment in the pretreated setting can serve as a predictive and prognostic marker [14]. The researchers assessed ctDNA over time in 52 patients with T790M-positive advanced NSCLC from the ASTRIS study who had progressed on EGFR TKI treatment. According to the analysis, patients with undetectable ctDNA at first follow-up within 50 days from the initiation of osimertinib therapy had significantly longer PFS and OS than those with detectable ctDNA (PFS, p = 0.022; OS, p = 0.009).
Moreover, the findings revealed the potential of ctDNA in the early detection of disease progression. Molecular progression occurred in 34 % of patients ahead of radiological progression, with an average lead time of 2.5 months. These patients were more likely to harbor copy number amplifications (CNAs) and TP53 mutations at the time of radiological progression, with the presence of CNAs indicating shortened PFS and OS compared to those without. Assessment of ctDNA clearance at first follow-up might be commendable considering these insights.
REFERENCES
- Nakagawa K et al., RELAY: A multicenter, double-blind, randomized phase 3 study of erlotinib in combination with ramucirumab or placebo in previously untreated patients with epidermal growth factor receptor mutation-positive metastatic non-small cell lung cancer. J Clin Oncol 37, 2019 (suppl; abstr 9000)
- Noronha V et al., Phase III randomized trial comparing gefitinib to gefitinib with pemetrexed-carboplatin chemotherapy in patients with advanced untreated EGFR mutant non-small cell lung cancer (gef vs gef+C). J Clin Oncol 37, 2019 (suppl; abstr 9001)
- Soria JC et al., Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378(2): 113-125
- Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci 2016; 107(9): 1179-1186
- Jänne PA et al., Antitumor activity of TAK-788 in NSCLC with EGFR exon 20 insertions. J Clin Oncol 37, 2019 (suppl; abstr 9007)
- Li Y et al., Afatinib in the treatment of advanced NSCLC with EGFR mutation: An observational real-world study. J Clin Oncol 37, 2019 (suppl; abstr e20518)
- Busto SA et al., Real-world clinical experience of the Galician Lung Cancer Group: Afatinib in patients with EGFR positive mutation. J Clin Oncol 37, 2019 (suppl; abstr e20654)
- Busto SA et al., Galician lung cancer group: Afatinib data as first-line treatment for elderly patients. J Clin Oncol 37, 2019 (suppl; abstr e20662)
- Kuan FC et al., Real-world outcome analysis of EGFR-mutated lung adenocarcinoma with brain metastases. J Clin Oncol 37, 2019 (suppl; abstr e13588)
- Pasello G et al., Clinical features and progression pattern of T790M+ compared with T790M-EGFR mutant NSCLC. J Clin Oncol 37, 2019 (suppl; abstr e20612)
- Thress KS et al., Complete clearance of plasma EGFR mutations as a predictor of outcome on osimertinib in the AURA trial. J Clin Oncol 2017; 35 (Suppl 15): 9018
- Shepherd FA et al., Early clearance of plasma EGFR mutations as a predictor of response to osimertinib in the AURA3 trial. J Clin Oncol 2018; 36 (Suppl 15): 9027
- Zhou C et al., Early clearance of plasma EGFR mutations as a predictor of response to osimertinib and comparator EGFR-TKIs in the FLAURA trial. J Clin Oncol 37, 2019 (suppl; abstr 9020)
- Song Y et al., Predictive and prognostic values of circulation tumor DNA clearance in osimertinib-treated advanced non-small cell lung cancer cohort. J Clin Oncol 37, 2019 (suppl; abstr 3036)
© 2019 Springer-Verlag GmbH, Impressum