Small-cell tumors: improvements in the second-line setting
Lurbinectedin monotherapy
Only limited therapeutic options are available for patients with relapsing small-cell lung cancer (SCLC). Topotecan is the only FDA-approved treatment for platinum-sensitive disease in the second-line setting. However, it induces merely modest clinical benefits, while at the same time giving rise to significant hematological toxicity.
A novel approach might result from the inhibition of deregulated oncogenic transcription factors. SCLC has been found to be a transcription-addicted tumor [1]. Rudin et al. described four molecular SCLC subtypes defined by the differential expression of four key transcription regulators [2]. Lurbinectedin, a selective inhibitor of oncogenic transcription, acts by binding DNA [3]. It not only targets tumor cells, inducing apoptosis, but also downregulates IL-6, IL-8, CCL2 and VEGF by inhibiting active transcription in tumor-associated macrophages [4].
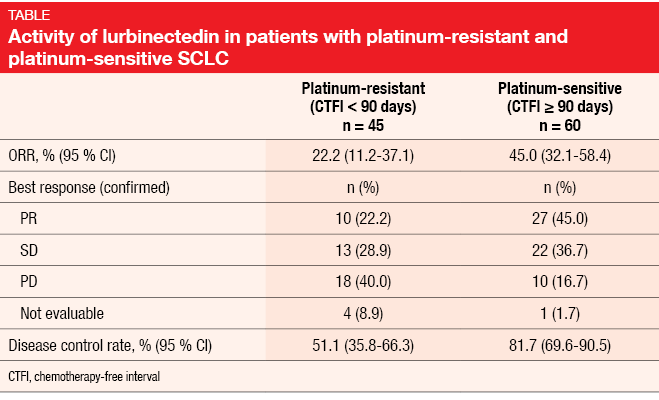
A single-arm, phase II basket trial investigated lurbinectedin monotherapy in nine different tumor types. Paz-Ares et at. presented the findings in patients with SCLC, who received lurbinectedin 3.2 mg/m2 every 3 weeks after one chemotherapy line [5]. Prior immunotherapy was allowed, whereas CNS involvement was not. A total of 105 patients entered the trial between October 2015 and October 2018 and were treated with a median of 4 cycles. Among these, 60 were defined as platinum-sensitive (i. e., chemotherapy-free interval ≥ 90 days) and 45 as platinum-resistant (chemotherapy-free interval < 90 days).
Encouraging findings in resistant disease
The antitumor activity of lurbinectedin was substantial, with an ORR of 35.2 % and a DCR of 68.6 %. Responses lasted for a median of 5.3 months. Compared to the platinum-sensitive group, the cohort with resistant disease showed lower ORRs (Table). However, these rates are still notable, which is important in a setting that lacks approved options. Patients with platinum-resistant disease experienced responses in 22.2 % and disease control in 51.1 %. Three of five patients with resistant SCLC and two of three with sensitive tumors in whom prior immunotherapy had failed achieved confirmed responses with lurbinectedin treatment. Duration of response was 4.7 and 6.2 months in the resistant and sensitive cohorts, respectively. Decreases in tumor size occurred in 65 % of the total population.
Median PFS was 3.9 months (2.6 and 4.6 months for resistant and sensitive patients, respectively), with a 6-month PFS rate of 33.6 % (18.8 % and 44.6 %, respectively). For OS, the median was 9.3 months in the overall cohort (5.0 and 11.9 months, respectively), and the 12-month survival rate amounted to 34.2 % (15.9 % and 48.3 %, respectively). Lurbinectedin showed a favorable and manageable safety profile. Among the non-hematological AEs, the most frequently reported AEs included fatigue, nausea, decreased appetite and vomiting. Neutropenia occurred as the most common hematological toxicity. The analysis revealed low rates of serious AEs (10.5 %) and AEs leading to treatment discontinuation (1.9 %). As the authors stated based on these observations, lurbinectedin emerges as a potential new treatment alternative for SCLC patients treated in the second line.
Carfilzomib plus irinotecan
A single-arm, phase II trial stratified by platinum sensitivity evaluated the combination of irinotecan (125 mg/m2 on days 1, 8 and 15 of a 28-day cycle) and the proteasome inhibitor carfilzomib in patients with extensive-disease SCLC progressing after one prior platinum-based regimen [6]. During the first cycle, carfilzomib was administered at a dose of 20mg/m2 on days 1 and 2, followed by 36 mg/m2 on all subsequent days (days 1, 2, 8, 9, 15 and 16 of a 28-day cycle). The rationale for the combined approach was the expected synergy of these drugs, as the inactivation of proteasome function allows for an increase in apoptosis and interference with Topo-I degradation. Overall, 62 patients participated; 25 of these were platinum-refractory (PR stratum), while 37 were platinum-sensitive (PS stratum). OS at 6 months was defined as the primary endpoint.
Irinotecan plus carfilzomib demonstrated effectivity in relapsed SCLC, with 6-month OS rates of 54 % and 59 % in the PR and PS strata, respectively. Median OS amounted to 6.8 and 6.9 months, respectively. Median PFS was 3.3 and 3.6 months, respectively. At 56.0 % and 67.6 %, disease control rates were comparable to those observed with other second-line agents, although CRs and PRs were lower in comparison (1.6 % and 16.1 %, respectively, for the total population). Assessments of the chymotrypsin-like activity (CLA) in peripheral blood mononuclear cells, which is a measure of the effect of carfilzomib on proteasome activity, revealed similar CLA declines in both platinum-sensitive and platinum-refractory patients. This suggests that proteasome inhibition did not account for the unanticipated success in the refractory group.
The safety profile of the combination resembled that of single-agent topotecan, amrubicin and irinotecan in relapsed SCLC. Forty-seven percent of patients experienced at least one grade-3 AE. Grade-4 toxicities occurred in 8 patients (12.9 %) and three (4.8 %) died, with two possible fatalities (i. e., myocardial infarction, lung infection) and one probable fatality (i. e., sepsis). According to the conclusion of the scientists, irinotecan plus carfilzomib is a viable option in relapsed SCLC and can be considered after progression on immunotherapy or in patients who cannot receive checkpoint inhibitors. However, due to toxicity, this regimen is not recommended for frail individuals with performance status > 2. The combination should be further explored in a confirmatory phase III trial.
Myelopreservation with trilaciclib
Despite the availability of rescue medications, there is still a significant unmet medical need in SCLC patients treated with topotecan as this drug causes severe myelosuppression in a significant percentage of cases. Neutropenia occurs in more than half of patients treated with topotecan at full dose, with a febrile neutropenia rate of approximately 3 % [7]. G-CSF rescue is frequently indispensable but often induces bone pain as a side effect [8]. Anemia and thrombocytopenia are observed in 31 % and 54 %, respectively, necessitating the use of erythropoiesis-stimulating agents or transfusions in many patients [7]. At the same time, dose reductions or schedule changes of topotecan have unknown effects on the efficacy of this treatment.
The CDK4/6 inhibitor trilaciclib is a first-in-class, potent, intravenous myelopreservation agent. It transiently blocks progression through the cell cycle, thereby preventing chemotherapy-associated damage in hematopoietic stem and progenitor cells. Dragnev et al. already demonstrated benefits of trilaciclib with respect to multi-lineage myelosuppression in extensive-stage SCLC patients receiving first-line chemotherapy [9]. The randomized, double-blind, placebo-controlled, phase II G1T28-03 study presented at ASCO 2019 tested trilaciclib in patients with extensive-stage SCLC receiving topotecan in the second or third-line setting [10]. In the experimental arm, 32 patients were treated with trilaciclib plus topotecan 1.5 mg/m2 until progression, while 29 patients received placebo plus topotecan in the control arm. Trilaciclib was administered intravenously on days 1 to 5 prior to topotecan.
Benefits without impaired efficacy
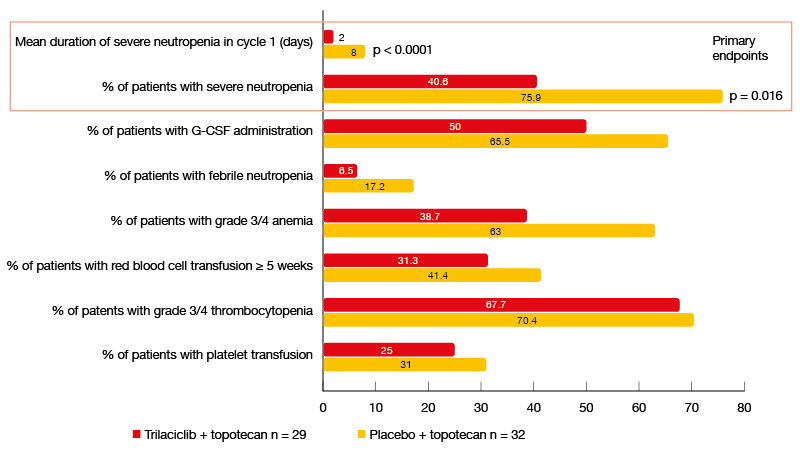
Indeed, the administration of trilaciclib made topotecan treatment safer and more tolerable. Compared to the placebo arm, the patients in the experimental arm completed more cycles and had fewer dose reductions. Myelopreservation benefits occurred across multiple lineages, with reductions in cytopenia rates and diminished necessity of rescue treatments (Figure). For the primary endpoints, i. e., mean duration of severe neutropenia in cycle 1 and occurrence of severe neutropenia, the analysis yielded significant differences in favor of the trilaciclib-treated group (p < 0.0001 and p = 0.016, respectively). Duration of severe neutropenia is a surrogate for an increased risk of febrile neutropenia, infection, intravenous antibiotic use and hospitalization.
Accordingly, the trilaciclib arm experienced fewer high-grade hematological toxicities, particularly neutropenia and anemia, and improved patient experience by decreasing the risk of deterioration during chemotherapy as compared to placebo, according to validated patient-reported outcome instruments. Benefits of trilaciclib were observed for general and physical wellbeing, quality-of-life measures specific for lung cancer patients, symptoms and impact of fatigue, and symptoms and effects on physical and functional wellbeing due to anemia.
At the same time, the use of trilaciclib did not impair the efficacy of chemotherapy. ORR, PFS and OS were comparable across the trilaciclib and placebo arms. Trilaciclib-related AEs of special interest were primarily low-grade and included headache, infusion-related reactions, and phlebitis. In their conclusion, the authors noted that these data extend the evidence for the clinical benefits of trilaciclib in SCLC as a first-in-class yelopreservation agent for patients treated with topotecan in the second- or third-line setting.
Figure: Myelopreservation benefits achieved with the administration of trilaciclib prior to topotecan in the second- or third-line setting
REFERENCES
- Christensen CL et al., Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell 2014; 26(6): 909-922
- Rudin CM et al., Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 2019; 19(5): 289-297
- Santamaria Nunez G et al., Lurbinectedin specifically triggers the degradation of phosphorylated RNA polymerase II and the formation of DNA breaks in cancer cells. Mol Cancer Ther 2016; 15(10): 2399-2412
- Belgiovine C et al., Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models. Br J Cancer 2017; 117(5): 628-638
- Paz-Ares L et al., Efficacy and safety profile of lurbinectedin in second-line SCLC patients: results from a phase II single-agent trial. J Clin Oncol 37, 2019 (suppl; abstr 8506)
- Arnold SM et al., Phase 2 study of carfilzomib plus irinotecan in small cell lung cancer progressing after prior platinum-based chemotherapy (NCT0194131). J Clin Oncol 37, 2019 (suppl; abstr 8513)
- on Pawel J et al., Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014; 32(35): 4012-4019
- Kirshner JJ et al., Prevention of pegfilgrastim-induced bone pain: a phase III double-blind placebo-controlled randomized clinical trial of the university of Rochester cancer center clinical community oncology program research base. J Clin Oncol 2012; 30(16): 1974-1979
- Dragnev KH et al., Trilaciclib decreases multi-lineage myelosuppression in extensive-stage small cell lung cancer (ES-SCLC) patients receiving 1st line chemotherapy. Ann Oncol 2018; 29 (suppl_8): viii596-viii602
- Hart LL et al., Effect of trilaciclib, a CDK 4/6 inhibitor, on myelosuppression in patients with previously treated extensive-stage small cell lung cancer receiving topotecan. J Clin Oncol 37, 2019 (suppl; abstr 8505)