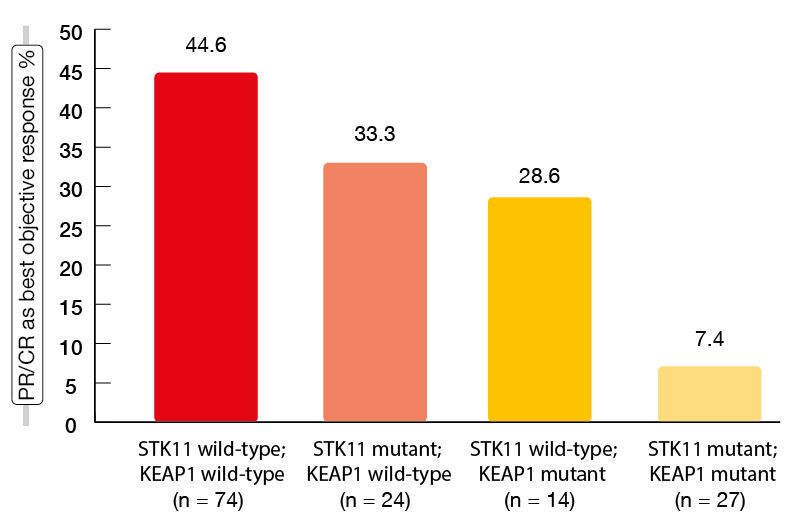Trial updates and new biomarkers in the field of immunotherapy
Long-term findings with pembrolizumab: KEYNOTE-001
KEYNOTE-001 was the first trial to demonstrate the activity of the PD-1 inhibitor pembrolizumab in patients with treatment-naïve or previously treated advanced NSCLC [1]. Notably, in this multicohort phase IB study, pembrolizumab showed greater activity with increasing PD-L1 tumor proportion score (TPS). Between May 2012 and July 2014, 550 patients with advanced NSCLC had been enrolled across 4 non-randomized and 2 randomized cohorts. Among these, 101 were treatment-naïve, while 449 had received previous treatment. The 5-year efficacy and safety outcomes of KEYNOTE-001 were reported by Garon et al. at ASCO 2019 [2]. At data cutoff, 100 patients were alive. The recent analysis represents the longest follow-up to date of pembrolizumab treatment in the setting of advanced NSCLC. In patients with treatment-naïve NSCLC, 23.2 % were alive at 5 years; in the pretreated cohort, this applied to 15.5 %. The authors noted that compared to this, the 5-year OS rate obtained in the United States using standard-of-care cytotoxic chemotherapies between 2008 and 2014 was 5.5 % [3]. In patients with PD-L1 TPS ≥ 50 %, the 5-year OS rates were 29.6 % and 25.0 % for the treatment-naïve and pretreated setting, respectively. Patients with TPS 1 % to 49 % showed lower 5-year survival rates (15.7 % and 12.6 %, respectively). ORRs in the total group amounted to 41.6 % and 22.9 % for treatment-naïve and pretreated patients, respectively, and DCRs were 83.2 % and 58.6 %. Forty-six out of 60 patients who received pembrolizumab treatment for ≥ 2 years were alive at data cutoff. Estimated 5-year OS rates in these 60 patients were 78.6 % and 75.8 % for the treatment-naïve and pretreated cohorts (n = 14 and 46, respectively). Objective responses occurred in 86 % and 91 %, respectively, with a median duration of response of 52.0 months and not reached, respectively. Updated safety data were consistent with the known profile of pembrolizumab. There was no evidence of cumulative immune-mediated toxicity or late-onset grade 3 to 5 toxicity. Overall, these data continue to demonstrate the potential of pembrolizumab treatment with respect to the improvement of long-term outcomes for treatment-naïve and pretreated patients with advanced NSCLC.
KEYNOTE-189: updated results & PFS2
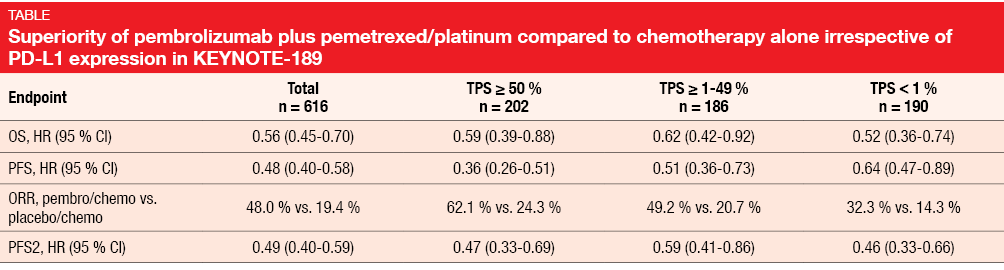
The randomized, double-blind, phase III KEYNOTE-189 trial demonstrated the superiority of first-line pembrolizumab combined with a pemetrexed/platinum doublet compared to placebo plus pemetrexed/platinum in metastatic non-squamous NSCLC [4]. Benefits were obtained concerning OS, PFS and ORR; at the same time, the safety profile proved manageable. Gadgeel et al. presented updated efficacy findings based on longer follow-up and, for the first time, PFS2, which is defined as the time from randomization to objective tumor progression on next-line treatment or death from any cause, whichever occurs first [5]. PFS2 can be used to quantify the impact of crossover on OS assessment and to determine whether treatment in one line positively or negatively affects the activity of the next line of therapy. In the ITT population, 410 patients received the pembrolizumab-based combination, whereas 206 were treated with placebo plus chemotherapy. At least one subsequent treatment had been administered in 44.6 % and 59.2 % of patients, respectively. Thirteen percent vs. 54 % had received ≥ 1 subsequent PD-1 or PD-L1 inhibitor. An in-study crossover took place for 40.8 % of patients treated in the control arm. Pembrolizumab plus pemetrexed and platinum continued to elicit a substantial survival benefit (median OS, 22.0 vs. 10.7 months; HR, 0.56). The 24-month OS rates were 45.5 % vs. 29.9 % for the two arms. Likewise, PFS was approximately doubled (9.0 vs. 4.9 months; HR, 0.48), with 24-month PFS rates of 20.5 % vs. 1.5 %. Moreover, the analysis revealed a substantial benefit of the pembrolizumab-based regimen with regard to PFS2 (17.0 vs. 9.0 months; HR, 0.49). ORRs were also higher in the experimental arm (48.0 % vs. 19.4 %). Benefits of the addition of pembrolizumab were observed for all of these endpoints despite the high rates of patients receiving subsequent therapies and performing in-study cross over, and regardless of PD-L1 expression (Table). After the prolonged follow-up, safety and tolerability of the pembrolizumab-based regimen remained manageable. According to the authors’ conclusion, these data confirm that pembrolizumab should be given as part of first-line therapy to maximize outcomes in patients with both PD-L1–expressing and PD-L1-non–expressing metastatic non-squamous NSCLC.
Significance of absolute PD-L1 levels
Predictive biomarkers for the optimal patient selection for immune checkpoint inhibitor treatment are still lacking, with PD-L1 expression remaining the main clinically applicable test. As part of a multicenter, retrospective study, Aguilar et al. analyzed patients with stage IV NSCLC and PD-L1 TPS of ≥ 50 % to answer the question of whether certain subsets within this range are more likely to benefit from PD-1 inhibitor treatment [6]. The entire cohort comprised 172 patients who received first-line pembrolizumab. Clinicopathological characteristics and clinical outcomes were compared among patients with PD-L1 TPS of 50 % to 74 % (n = 68) vs. 75 % to 100 % (n = 104), and 50 % to 89 % (n = 99) vs. 90 % to 100 % (n = 73). Indeed, the findings demonstrated that higher PD-L1 TPS levels of ≥ 75 % and ≥ 90 % are associated with improved clinical outcomes. After adjustment for never smokers, squamous histology and mutation status, these patients were shown to derive greater survival benefit than their counterparts with lower PD-L1 expression levels (HRs, 0.63 and 0.50, respectively). The comparisons also yielded significant PFS prolongation for both PD-L1 75 % to 100 % vs. 50 % to 74 % (HR, 0.61) and 90 % to 100 % vs. 50 % to 89 % (HR, 0.52). Similarly, ORRs were in favor of the populations with higher PD-L1 expression. Responders had higher PD-L1 TPS than non-responders. The mean TPS in patients achieving partial or complete response was 82.1 %; in those who showed stable and progressive disease, this was 73.7 % (p = 0.001). The investigators noted that these results should be taken into consideration when deciding between first-line pembrolizumab monotherapy and pembrolizumab plus platinum-doublet chemotherapy. Also, they deserve attention in the context of the design and interpretation of clinical trials for NSCLC with PD-L1 TPS ≥ 50 %.
Additional markers: STK11 and KEAP1
STK11/LKB1 genomic alterations have been found to be a mediator of “cold” tumor immune microenvironment and a major driver of primary resistance to PD-1 inhibition in non-squamous NSCLC [7]. STK11 is one of the most frequently inactivated tumor suppressor genes in this disease. It codes for the protein LBK1 that has a role in the regulation of cellular growth and metabolism. Moreover, the KEAP1 gene is genetically and functionally linked to STK11, and the two genes are frequently co-mutated [8, 9]. The retrospective, international study conducted by Skoulidis et al. addressed the effect of these markers as molecular determinants of clinical outcomes obtained with pembrolizumab plus pemetrexed and platinum in the first-line setting of metastatic non-squamous NSCLC [10]. STK11and KEAP1 genomic alterations were shown to be significantly associated with poor outcomes with chemoimmunotherapy. This applied to each of the alterations but particularly to the co-mutated setting. Median PFS was 8.4 months for the double wild-type population, but only 2.7 months for those with double mutants (p < 0.0001); for median OS, this was 20.4 vs. 6.6 months (p = 0.005).Likewise, ORRs showed gradual worsening when viewed as a function of an increasing number of mutations (Figure 1). In the group of patients with primary refractory disease, as many as 76.5 % had STK11 and/or KEAP1 alterations. Also, the presence of these mutations correlated with a lack of apparent PFS or OS benefit from the addition of pembrolizumab to pemetrexed plus platinum. The negative impact of STK11 and KEAP1 alterations on clinical outcomes with chemoimmunotherapy was most prominent in patients with high tumor mutational burden and PD-L1–positive tumors. At the same time, in patients with STK11– and/or KEAP1-mutant tumors, tumor mutational burden and PD-L1 expression did not affect the outcomes. Based on these findings, the authors proposed the integration of STK11 and KEAP1 mutations into a composite genomic marker of poor clinical outcome with chemoimmunotherapy. This would capture a subgroup of approximately 25 % of NSCLC patients with an unmet need for novel strategies to establish effective anti-tumor immunity.
Figure 1: Objective response rates obtained with pembrolizumab plus chemotherapy in STK11– and KEAP1-defined subgroups
NLR, PLR, and LDH
Russo et al. demonstrated that easily determinable parameters including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lactate dehydrogenase (LDH) might contribute to patient selection for immunotherapy [11]. The investigators assessed dynamic changes of these inflammation markers over time and the outcomes in 71 consecutive NSCLC patients treated with nivolumab or pembrolizumab. NLR ≥ 5, PLR ≥ 200, and LDH levels ≥ upper normal limit (UNL) were considered high. Indeed, NLR ≥ 5 was associated with lower PFS and OS, with increasing predictive value from baseline to week 12. PLR ≥ 200 at baseline and week 12 significantly correlated with shorter OS but not PFS. For LDH levels ≥ UNL at baseline, the analysis showed an association with shorter PFS and OS; reductions in LDH levels at 12 weeks compared with baseline values conferred OS improvement. The researchers summarized that baseline levels for NLR, PLR and LDH as well as dynamic changes of LDH levels at 12 weeks significantly predict outcomes in patients treated with single-agent immune checkpoint inhibitors.
Does autoimmune disease preclude treatment?
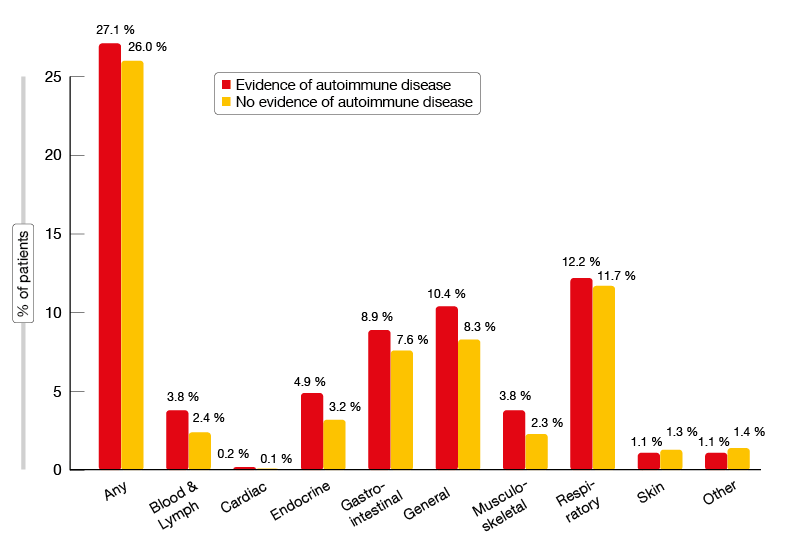
Patients with a history of autoimmune disease are usually excluded from clinical trials testing immunotherapeutic approaches. However, anecdotal and early evidence suggests that immune checkpoint inhibitors are being used in routine care for the treatment of advanced NSCLC even in such patients [12]. Based on these observations, a retrospective observational cohort study was conducted to describe the real-world characteristics and outcomes including AEs in patients with advanced NSCLC with and without a prior history of autoimmune disease who had received at least one dose of an approved immune checkpoint inhibitor in 49 predominantly community-based oncology practices in the USA [13]. Local treatment including surgery and chemoradiation in stage III disease within one year prior to the initiation of immunotherapy represented an exclusion criterion. The records of 2,402 patients were included in the analysis. Twenty-two percent of these (n = 531) had a history of autoimmune disease. Compared to the cohort without a history of autoimmune disease, they showed similar patient and disease characteristics except for a higher proportion of females (54.6 % vs. 43.5 %). The investigators noted that patients with a history of autoimmune disease had similar efficacy outcomes compared to those without. For OS, real-world PFS, time to treatment discontinuation and time to next treatment, the Kaplan-Meier curves were superimposable, and statistics did not yield any significant differences. With respect to tolerability, patients with a history of autoimmune disease demonstrated an increased incidence of immune-related AEs. This was especially true for endocrine, gastrointestinal, blood and lymphatic disorders, as well as general disorders (Figure 2). Further research is needed to improve understanding of the impact of autoimmune disease on the incidence of immune-related AEs and patient outcomes.
Figure 2: Incidence of immune-related AEs in immune-checkpoint-inhibitor–treated patients with and without a history of autoimmune disease
REFERENCES
- Garon EB et al., Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372(21): 2018-2028
- Garon EB et al., 5-year long-term overall survival for patients with advanced NSCLC treated with pembrolizumab: results from KEYNOTE-001. J Clin Oncol 37, 2019 (suppl; abstr LBA9015)
- Leighl NB et al., Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med 2019; 7(4): 347-357
- Gandhi L et al., Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378(22): 2078-2092
- Gadgeel S et al., KEYNOTE-189: updated overall survival and progression after the next line of therapy with pembrolizumab plus chemotherapy with pemetrexed and platinum vs placebo plus chemotherapy for metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 37, 2019 (suppl; abstr 9013)
- Aguilar EJ et al., Comparison of outcomes with PD-L1 tumor proportion score (TPS) of 50-74% vs 75-100% in patients with non-small cell lung cancer (NSCLC) treated with first-line PD-1 inhibitors. J Clin Oncol 36, 2018 (suppl; abstr 9037)
- Skoulidis F et al., STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 2018; 8(7): 822-835
- Skoulidis F et al., Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 2015; 5(8): 860-877
- Galan-Cobo A et al., LKB1 and KEAP1/NRF2 pathways cooperatively promote metabolic reprogramming with enhanced glutamine dependence in KRAS-mutant lung adenocarcinoma. Cancer Res 2019 Apr 30. pii: canres.3527.2018. doi: 10.1158/0008-5472.CAN-18-3527. [Epub ahead of print]
- Skoulidis F et al., Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non-squamous non-small cell lung cancer. J Clin Oncol 37, 2019 (suppl; abstr 102)
- Russo A et al., Dynamic changes of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lactate dehydrogenase (LDH) during treatment with immune checkpoint inhibitors (ICIs) in non-small cell lung cancer (NSCLC). J Clin Oncol 37, 2019 (suppl; abstr 2596)
- Darvin P et al., Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018; 50(12): 165
- Khozin S et al., Real-world outcomes of patients with advanced non-small cell lung cancer (aNSCLC) and autoimmune disease receiving immune checkpoint inhibitors. J Clin Oncol 37, 2019 (suppl; abstr 110)
© 2019 Springer-Verlag GmbH, Impressum