NSCLC with MET alterations: molecular insights and innovative treatments
Oncogenic alterations of the exon 14 of the mesenchymal-epithelial transition (MET) gene occur in 3 % to 4 % of patients with adenocarcinoma of the lung and in 2 % of those with squamous-cell lung cancer [1, 2].
MET exon 14 (METex14) mutations tend to coexist with MET amplifications. Multiple agents are in development for the treatment of lung cancer patients with these alterations. The highly selective, oral MET tyrosine kinase inhibitors (TKIs) capmatinib and tepotinib have already gained regulatory approval.
Capmatinib is being investigated in patients with stage IIIB/IV non–small-cell lung cancer (NSCLC) and METex14 skipping mutations or MET amplifications in the ongoing, international, open-label, phase II GEOMETRY mono-1 study. This trial has revealed rapid, deep, and durable responses with capmatinib when administered under fasting conditions in patients harboring METex14 skipping mutations [3]. Based on this, capmatinib has received accelerated approval by the US Food and Drug Administration for the treatment of patients with METex14-mutant metastatic NSCLC in May 2020.
GEOMETRY mono-1: high-level MET-amplified NSCLC
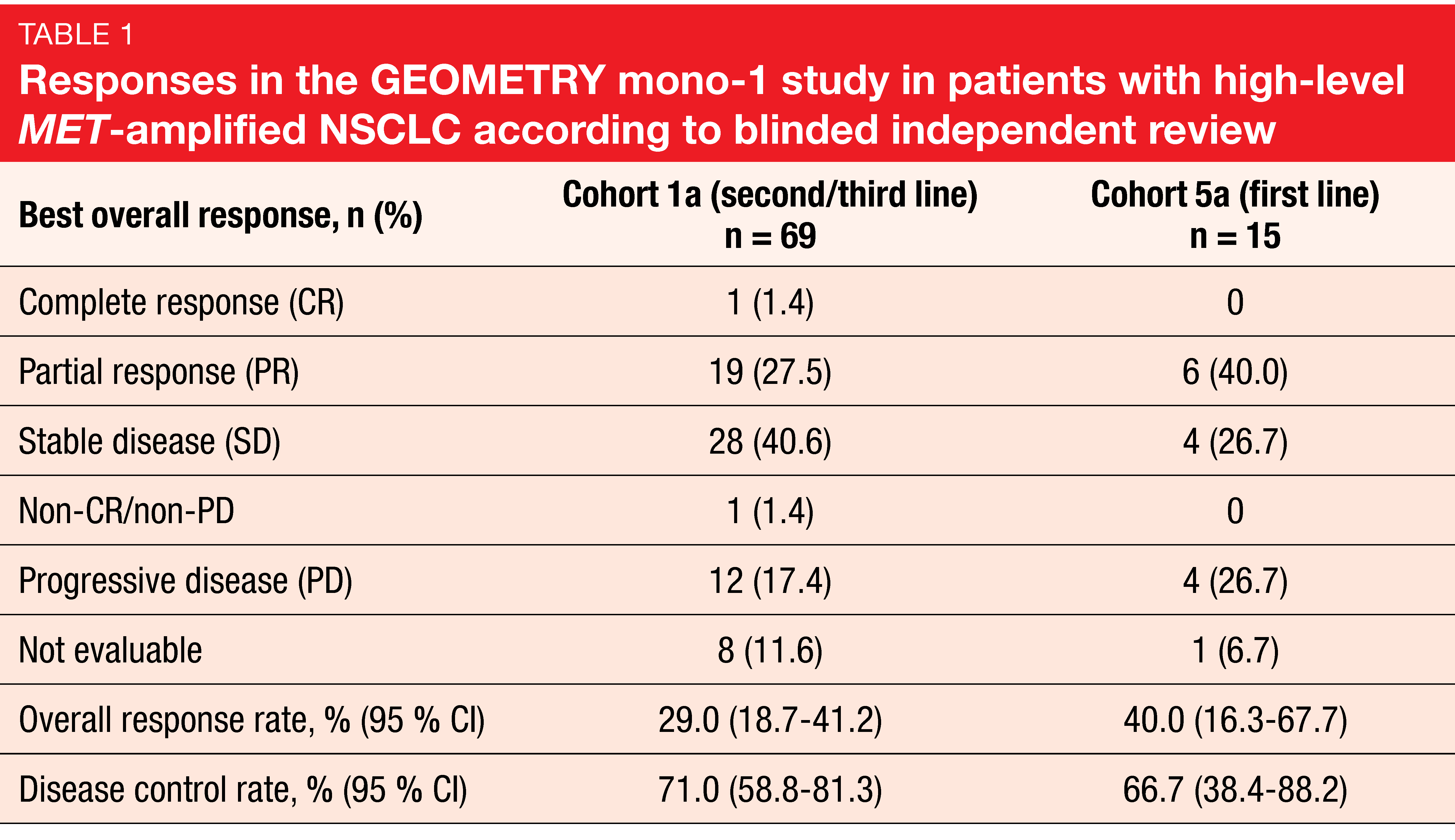
High-level MET amplification (i.e., gene copy number ≥ 10) has emerged as a potential predictive biomarker for MET-directed therapies. At the ASCO 2020 Congress, Wolf et al. reported the results for capmatinib 400 mg BID (twice daily) in patients included in GEOMETRY mono-1 who had high-level MET-amplified NSCLC without METex14 mutations [4]. These were either pretreated with one or two lines of systemic therapy (Cohort 1a; n = 69), or treatment-naïve (Cohort 5a; n = 15). Compared to patients with METex14 mutations, who are predominantly female and never smokers, this population with high-level MET amplifications tended to be male and to have a history of smoking. Overall response rate (ORR) was defined as the primary endpoint. According to blinded independent review, ORRs were 29.0 % and 40.0 % for Cohorts 1a and 5a, respectively (Table 1). One patient in Cohort 1a achieved complete response. Disease control was obtained in 71.0 % and 66.7 %, respectively. The treatment line appeared to determine ORR but not the other outcomes, which were fairly similar across cohorts. Responses lasted for a median of approximately 8 months for both pretreated and treatment-naïve patients. Progression-free survival (PFS) was 4.07 and 4.17 months, respectively, and overall survival (OS) was 10.61 and 9.56 months, respectively. Capmatinib showed a favorable safety profile that matched previous reports. The majority of treatment-related adverse events (AEs) were grades 1 and 2.
The authors concluded that the analysis demonstrated evidence of activity of capmatinib in patients with high-level MET-amplified advanced NSCLC, although response rates were moderate compared to those achieved in the first and second/third treatment lines in the METex14-mutated cohorts of GEOMETRY mono-1 (67.9 % and 40.6 %, respectively) [6]. It can be assumed that a subgroup within the high-level MET-amplified population derives distinct benefit from MET-directed therapy. This group should be characterized more precisely in the future.
Capmatinib use without fasting restrictions
Efficacy and safety results from Cohort 6 of the GEOMETRY mono-1 study were presented by Groen et al. [5]. This expansion group received capmatinib 400 mg BID in the second-line setting and was the first cohort not to include fasting restrictions. It included patients with high-level MET amplification and no METex14 mutations (group 1; n = 3) and METex14 mutations with any MET gene copy number (group 2; n = 31).
Only patients in group 2 responded, with an ORR of 48.4 % based on partial responses according to blinded independent review, although all of the patients included in group 1 achieved disease stabilization. Duration of response in group 2 was 6.93 months. Median PFS was 8.11 months in group 2 and not evaluable in group 1 due to the limited number of patients. Overall, the safety profile proved manageable and was consistent with the safety profile observed under fasting conditions. Notably, there was a numerical trend towards fewer gastrointestinal AEs of any grade when capmatinib was taken without fasting restrictions compared to the administration during a fasted state.
In their summary, the authors concluded that capmatinib demonstrated efficacy as a second-line agent. Taken together with previously reported results, the activity of capmatinib was confirmed irrespective of the line of treatment, with higher ORR in earlier lines.
MET-directed antibody mixture
Sym015 is a synergistic mixture of two recombinant humanized monoclonal antibodies against non-overlapping epitopes of MET. This antibody approach was developed to improve MET selectivity avoiding off-target toxicity and to circumvent intracellular acquired resistance mechanisms to MET TKIs, such as kinase domain mutations. In the phase IIa setting, Sym015 was tested in a total of 45 patients at a loading dose of 18 mg/kg on day 1 of cycle 1 followed by a maintenance dose of 12 mg/kg two-weekly [6]. Twenty of these patients had NSCLC with MET amplifications or METex14 deletions. In this cohort, treatment with prior MET- and/or EGFR-targeting agents was permitted, with 10 patients each being MET-TKI–naïve and MET-TKI–pretreated. Each of these groups contained both patients with MET amplifications and METex14 deletions.
Responses in lung cancer patients occurred in treatment-naïve individuals only (n = 5; 25 %), with a median duration of 13.8 months. None of the pretreated patients developed complete or partial responses, although the data suggest minor responses and prolonged stabilization of disease in some cases. The disease control rate (DCR) was 100 % for MET-TKI-naïve patients and 60 % for the MET-TKI–pretreated ones. Median OS had not been reached yet in the overall NSCLC cohort, and PFS was 7.4 vs. 5.4 months. The response rate obtained in the MET-TKI–naïve population was similar to that observed with MET TKI treatment in METex14-mutant and MET-amplified NSCLC.
Sym015 showed a favorable safety profile, with peripheral edema, aspartate aminotransferase increases, nausea, asthenia and decreased appetite constituting the most common treatment-related AEs. Six patients in the overall population of 45 individuals experienced grade ≥ 3 AEs, but only one lung cancer patient required a dose reduction. No patient discontinued treatment due to AEs. Moreover, the analysis suggested that liquid biopsy is a viable option for the selection of patients with METex14 deletions, as there was a 100 % concordance between local tumor and blood circulating tumor DNA (ctDNA) for this aberration in nine evaluable patients. On the other hand, the concordance for the detection of MET amplification was low at 29 %, which might be due to factors such as low tumor shedding or tumor evolution. Evaluation of Sym015 in combination with MET TKI treatment to delay or treat resistance is planned.
Subtypes of METex14 alterations
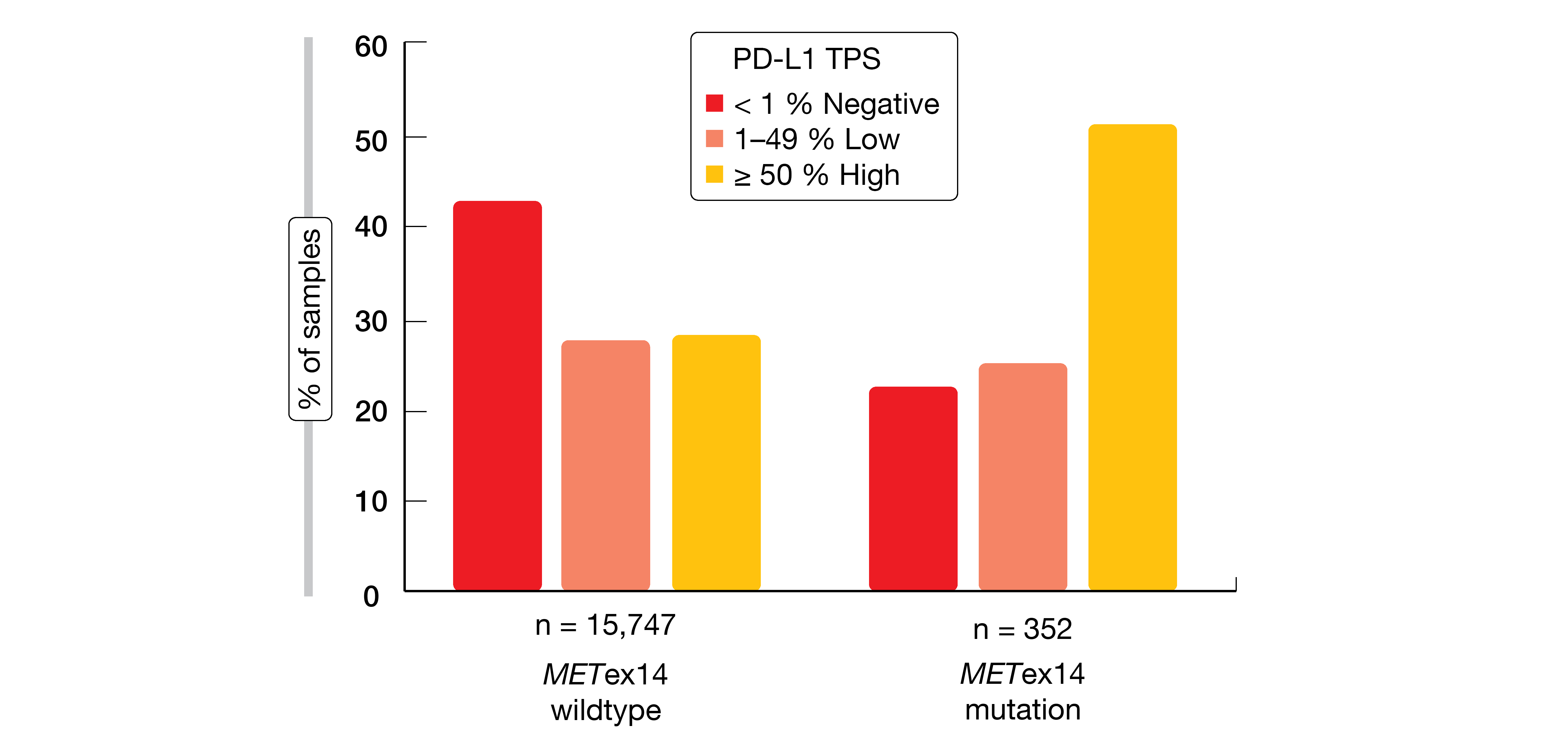
Exon 14 skipping is caused by a range of genomic alterations in exon 14 and its flanking introns. Awad et al. analyzed samples from NSCLC patients to characterize potential differences across various METex14 alteration subtypes and to assess co-occurring alterations as well as immunotherapy biomarkers that might impact treatment efficacy and inform combination strategies [7]. NGS-based hybrid-capture genomic profiling of tumor DNA from 60,495 NSCLC patients revealed METex14 alterations in 2.3 % (n = 1,387) at multiple functional site subsets resulting in exon 14 skipping, deletion, or mutation at Y1003. METex14-altered lung tumors showed significantly lower tumor mutational burden (TMB) than those with METex14 wildtype (p < 0.001). Moreover, they were enriched for high (≥ 50 %) PD-L1 expression compared with wildtype samples (48 % vs. 29 %; Figure 1). PD-L1 positivity was relatively similar across METex14 alteration functional site subsets. None of the cases demonstrated an association between TMB and PD-L1 expression. Additional data are required to determine the predictive role of these biomarkers for immunotherapy response.
Also, the frequency of co-alterations such as MDM2, CDK4 and MET amplification was largely consistent across splicing functional sites. Concurrent drivers including KRAS and EGFR mutations were rare at 3.2 % and 0.65 %, respectively. No concurrent BRAFV600E mutations or ALK/ROS1/NTRK fusions emerged.
According to the analysis of 36 paired cases, potential acquired resistance mechanisms appeared to be essentially independent of the primary METex14 alteration subtype. Resistance alterations included recurrent secondary MET mutations (25 % of pairs), MET amplification (8 % of pairs), and individual cases with EGFR/ErbB2 activation, KRAS amplification, and PI3K mutation.
DNA- vs. RNA-based assays
Assuming that DNA-based assays alone might be suboptimal for the detection of METex14 mutations, Jurkiewicz et al. examined profiling data of lung adenocarcinomas determined by NGS to compare the performance of DNA- and RNA-based assays for the detection of METex14 variants [8]. The tumors of 644 patients were profiled by a custom targeted DNA-based panel that targets MET exons 2, 14, 16, 18, and 19. Cases without DNA-based driver mutations were reflexed to an NGS-based RNA fusion panel.
Over a 21-month period, DNA profiling detected METex14 skipping events in 2.5 % of patients. However, RNA analysis of driver-negative cases identified nine additional METex14 mutations, which made for a total of 3.9 % events. Thus, 36 % of METex14 mutations were missed by the DNA panel. The variants identified only by the RNA panel tended to be present at the intron 13 splice acceptor site or other sites relevant to splicing. These were not covered by the DNA panel, while the intron 14 splice donor site was. The authors noted that mutations can occur in the splice acceptor site, branching site A and polypyrimidine tracks. Custom DNA panels that cover these areas could increase assay sensitivity but require deeper intronic coverage, which poses a technical challenge.
Overall, DNA-based NGS panels can potentially miss METex14 skipping events in lung adenocarcinomas when the panel primers do not target both the 3´ splice site of intron 13 and the 5´ splice site of intron 14. A reflex workflow testing for RNA fusions in cases without DNA-detected driver mutations can potentially capture such events. With respect to future research, histological, clinical and molecular characterization of the variants detected only by RNA assays warrants further exploration.
The VISION trial: tepotinib
The phase II VISION trial assessed the efficacy and tolerability of the MET TKI tepotinib at a daily dose of 500 mg in patients with locally advanced or metastatic NSCLC harboring METex14 skipping mutations after ≤ 2 lines of therapy. Based on these results, tepotinib and its companion diagnostic were approved in Japan in March 2020. MET alterations had been detected through liquid biopsy or tissue biopsy before trial inclusion. Patients with asymptomatic brain metastases were allowed to enroll. Le et al. reported the primary efficacy, safety, and biomarker results of the VISION trial [9].
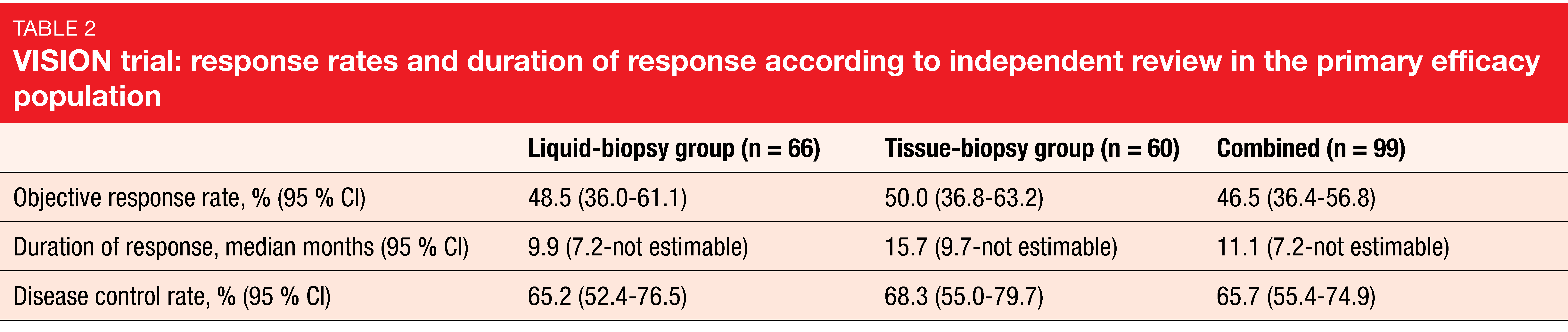
ORR according to independent review, which was defined as the primary endpoint, was 48.5 % in the liquid-biopsy–positive group, 50.0 % in the tissue-biopsy–positive group, and 46.5 % in the combined group that was liquid- and/or tissue-biopsy–positive (Table 2). Tumor shrinkage occurred in 89 % of all patients. In the combined group, median PFS and OS were 8.5 and 17.1 months, respectively. Outcomes in patients with baseline brain metastases (n = 11), all of which were non-target lesions, were comparable to those in the overall population, with an ORR of 54.5 % and median PFS of 10.9 months.
Sixty-seven percent of patients achieved molecular ctDNA responses, i.e., reductions in METex14 mutant allele frequency. Among these, high response rates were observed, with 71 % and 88 % experiencing radiographic response and disease control, respectively. Tepotinib had a manageable tolerability profile. Peripheral edema, nausea and diarrhea were the most common AEs. Grade ≥ 3 treatment-related AEs occurred in 27.6 % of patients. Dose reductions became necessary in 32.9 %, and permanent discontinuations in 11.2 %.
The authors concluded that tepotinib is a promising targeted therapy with durable clinical activity in NSCLC patients with METex14 skipping mutations identified by liquid or tissue biopsy.
Quality-of-life data from VISION
Findings on health-related quality of life in the VISION trial were reported separately at the ASCO Congress [10]. This outcome was assessed using the EORTC QLQ-LC13, EORTC QLQ-C30 and EQ-5D-5L questionnaires, as well as the Visual Analog Scale (VAS). At study entry, almost all patients had metastatic disease; they were older than patients with other actionable molecular alterations (median age, 74.0 years), and the majority had an ECOG performance status of 1. Baseline scores showed moderate-to-high functioning and quality of life, and a moderate lung cancer symptom burden.
For the QLQ-LC13 symptoms, mean changes from baseline indicated a meaningful improvement in cough and numerical improvement in dyspnea and chest pain. Mean changes in the QLQ-C30 global health and functional scale scores and EQ-5D-5L VAS scores demonstrated stability in quality of life over time. These findings, together with the efficacy and safety results from the VISION study, support tepotinib as a promising treatment option in NSCLC patients with METex14 skipping mutations.
Robust activity of savolitinib
A Chinese multicenter, single-arm phase II study evaluated the highly selective, oral MET TKI savolitinib in patients with unresectable or metastatic METex14-skipping–positive pulmonary sarcomatoid carcinoma (PSC; n = 25) and other types of NSCLC (n = 45) [11]. Patients were unfit for chemotherapy or had not responded to it. PSC is a rare type of NSCLC with particularly aggressive clinical behavior and poor prognosis that is often resistant to chemotherapy. Savolitinib was prescribed in a weight-adjusted manner, with daily doses of 600 mg and 400 mg for patients weighing ≥ 50 kg and < 50 kg, respectively.
Savolitinib showed robust and durable activity with an ORR of 49.2 % in the efficacy-evaluable set. Responses lasted for a median of 9.6 months. Median PFS was 6.9 months; patients with PSC showed shorter PFS than those with other NSCLC types (5.5 months and 9.7 months, respectively). Also, PFS was longer in the previously treated group (13.8 months) than in the treatment-naïve cohort (5.6 months), although this reflects the fact that nearly half of patients in the treatment-naïve cohort had PSC. Median OS was 14.0 months.
Treatment-related serious AEs including hepatic dysfunction, drug hypersensitivity and pyrexia occurred in 25.7 % of patients. One patient died of tumor lysis syndrome. Treatment discontinuation due to AEs became necessary in 14.3 %. Overall, savolitinib demonstrated promising anti-tumor activity and acceptable tolerability.
Characteristics of early-stage METex14-mutant lung cancer
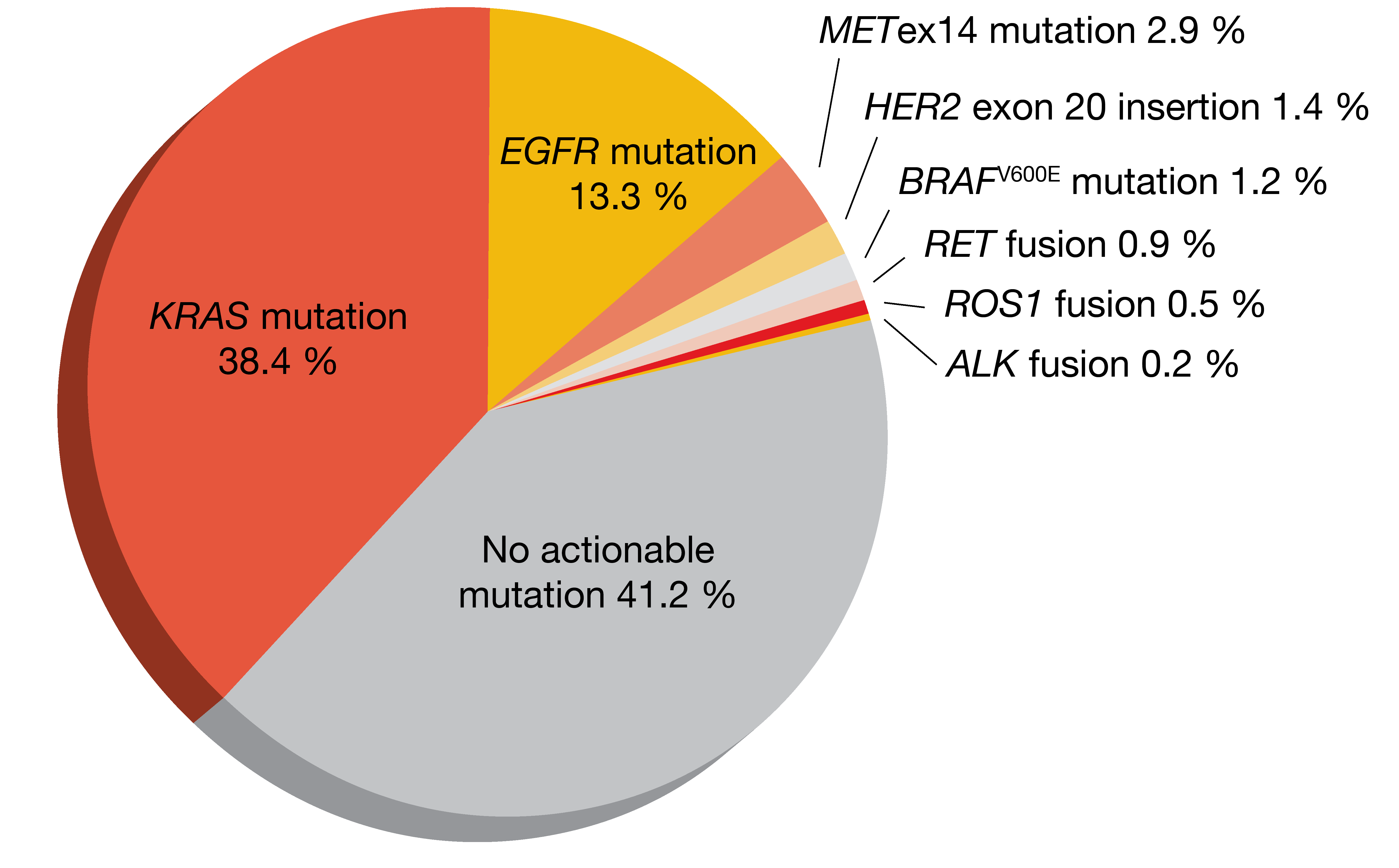
Clinical and genomic features of METex14-mutant NSCLC have been characterized in the metastatic setting, while less is known about this molecular subtype in early-stage disease. Therefore, Recondo et al. retrospectively assessed various features of METex14-mutant lung cancer in a cohort of 613 patients with resected stage I-III NSCLC and compared them to stage IV lung cancer [12]. The prevalence of METex14 mutations was 2.8 % in this group; non-squamous tumors showed a higher frequency (2.9 %; Figure 2) than those with squamous histology (1.4 %).
Regarding genomic co-alterations, MET amplifications, TP53 mutations and CDKN2A/B loss were significantly less prevalent in stages I-III than in stage IV NSCLC. The difference for MDM2 and CDK4/6 amplification was not significant, while KRAS mutation/amplification and EGFR mutation/amplification occurred in stage IV tumors only. High PD-L1 expression with tumor proportion scores (TPS) of ≥ 50 % was infrequent in stages I and II (13.5 % and 14.3 %, respectively) but considerably more prevalent in stage III (36.0 %), although this was still lower than the prevalence of PD-L1 TPS ≥ 50 % observed in stage IV (48.7 %).
With respect to clinical outcomes, the analysis showed that approximately 46 % of patients with stage II or III disease experienced recurrence after resection with curative intent. Median disease-free survival (DFS) from surgery in these groups was only 2.6 and 2.1 years, respectively. On the other hand, DFS for patients with stage I disease was 8.3 years (p = 0.017). The investigators emphasized that clinical trials exploring the role of adjuvant and neoadjuvant MET-targeted therapy in this population might be warranted.
REFERENCES
- Drilon A et al., Targeting MET in lung cancer: will expectations finally be MET? J Thorac Oncol 2017; 12: 15-26
- Tong JH et al., MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res 2016; 22: 3048-3056
- Wolf J et al., Capmatinib (INC280) in METΔex14-mutated advanced non-small cell lung cancer (NSCLC): Efficacy data from the phase II GEOMETRY mono-1 study. J Clin Oncol 37, 2019 (suppl; abstr 9004)
- Wolf J et al., Capmatinib in patients with high-level MET-amplified advanced non-small cell lung cancer (NSCLC): results from the phase 2 GEOMETRY mono-1 study. J Clin Oncol 38: 2020 (suppl; abstr 9509)
- Groen HJM et al., Capmatinib in patients with METex14-mutated or high-level MET-amplified advanced non-small-cell lung cancer (NSCLC): results from cohort 6 of the phase 2 GEOMETRY mono-1 study. J Clin Oncol 38: 2020 (suppl; abstr 9520)
- Camidge DR et al., Safety and preliminary clinical activity of the MET antibody mixture Sym015 in advanced non-small cell lung cancer (NSCLC) patients with MET amplification/exon 14 deletion (METAmp/Ex14Δ). J Clin Oncol 38: 2020 (suppl; abstr 9510)
- Awad M et al., Characterization of 1,387 NSCLCs with MET exon 14 (METex14) skipping alterations (SA) and potential acquired resistance (AR) mechanisms. J Clin Oncol 38: 2020 (suppl; abstr 9511)
- Jurkiewicz M et al., Efficacy of DNA vs. RNA NGS based methods in MET Exon 14 skipping mutation detection. J Clin Oncol 38: 2020 (suppl; abstr 9036)
- Le X et al., Primary efficacy and biomarker analyses from the VISION study of tepotinib in patients with NSCLC with MET exon 14 skipping. J Clin Oncol 38: 2020 (suppl; abstr 9556
- Paik PK et al., Tepotinib in NSCLC patients with MET exon 14 skipping: health-related quality of life. J Clin Oncol 38: 2020 (suppl; abstr 9575)
- Lu S et al., Phase II study of savolitinib in patients with pulmonary sarcomatoid carcinoma and other types of non-small cell lung cancer harboring MET exon 14 skipping mutations. J Clin Oncol 38: 2020 (suppl; abstr 9519)
- Recondo G et al., Clinical characteristics, genomic features, and recurrence risk of early-stage MET exon 14 mutant non-small cell lung cancer (NSCLC). J Clin Oncol 38: 2020 (suppl; abstr 9042)