Small-cell lung cancer: moving the limits further
High-dose irradiation proves feasible
Concurrent chemotherapy and thoracic radiotherapy (TRT) have been the standard treatment for limited-stage small-cell lung cancer (SCLC) since the early 1990s, with twice-daily TRT at a dose of 45 Gy being the most commonly recommended schedule. However, less than one third of patients are cured after chemoradiotherapy. In up to 50 %, local failure occurs that is associated with inferior survival [1, 2]. Hallqvist et al. showed that high-dose, twice-daily TRT of 60 Gy is feasible and safe [3]. Based on the hypothesis that this strategy is tolerable and improves local control and survival, the randomized phase II trial by Grønberg et al. compared 60 Gy in 40 fractions with 45 Gy in 30 fractions twice daily (10 fractions per week) [4]. Four courses of chemotherapy with cisplatin or carboplatin plus etoposide (EP) were administered in weeks 0, 3, 6, and 9. All patients started TRT along with the second chemotherapy course. Prophylactic cranial irradiation (PCI) was offered to anyone who responded to chemoradiotherapy after the fourth chemotherapy cycle. Patients with stage II/III or inoperable stage I who had not received any prior systemic therapy or TRT were enrolled, with 84 and 76 individuals analyzed in the high-dose and standard-dose arms, respectively. Two-year survival rates constituted the primary endpoint of the study.
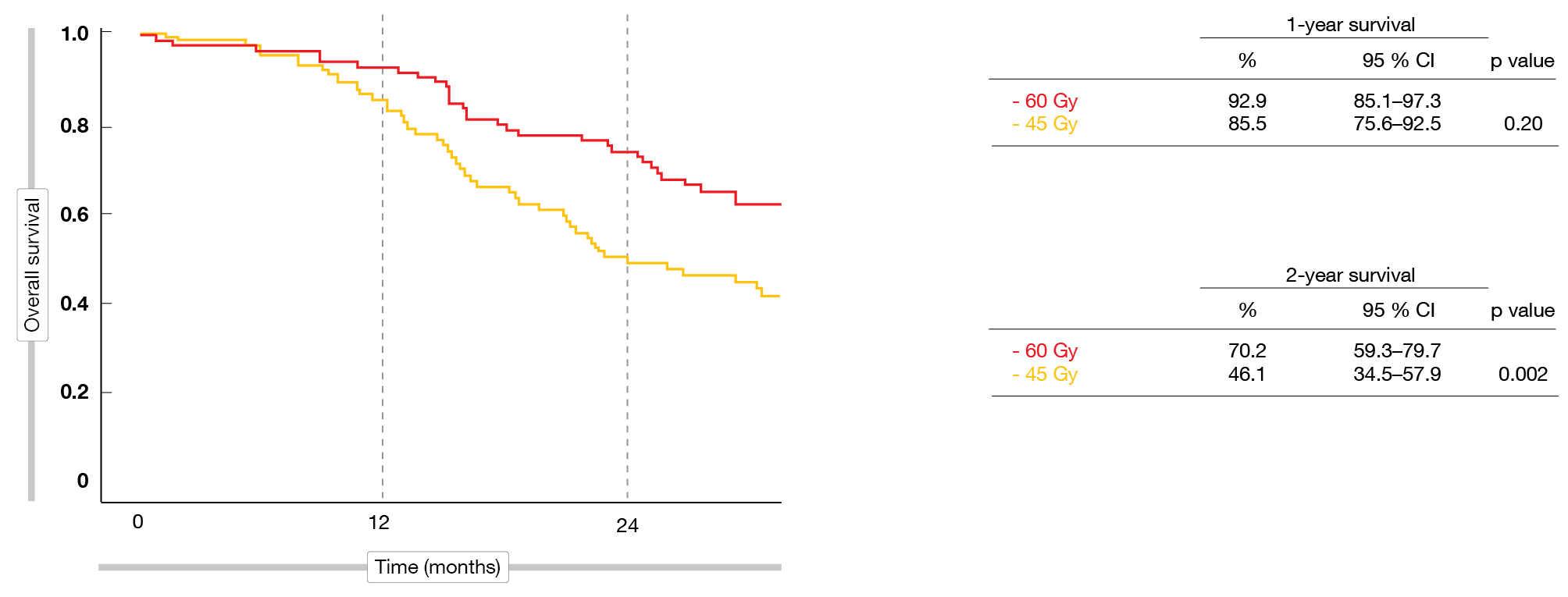
Delivering 60 Gy proved feasible in almost all cases. In both arms, 96 % of patients completed TRT, and more than 80 % received PCI. Objective responses were similar with high-dose and standard-dose TRT (88.5 % and 84.9 %, respectively). This also applied to complete responses (20.5 % vs. 23.2 %). Regarding the primary endpoint, the analysis demonstrated a significant and substantial improvement with the high-dose regimen: at 2 years, OS rates were 70.2 % vs. 46.1 % (p = 0.002; Figure 1). Median OS differed significantly (41.6 vs. 22.9 months; HR, 0.63; p = 0.027), whereas PFS did not (19.9 vs. 14.4 months; HR, 0.80; p = 0.257).
Importantly, the higher dose did not cause more radiotoxicity than the standard dose. Grade-3/4 AE rates for cytopenia, neutropenic infections, esophagitis and pneumonitis were similar across the treatment arms.
Figure 1: Survival after 1 and 2 years with thoracic irradiation at doses of 60 Gy vs. 45 Gy in limited-stage SCLC
ES-SCLC: new findings from the CASPIAN trial
After more than three decades of limited progress in extended-stage SCLC (ES-SCLC), the addition of immunotherapy to platinum-based chemotherapy has improved OS in the first-line setting [5, 6]. The global, open-label, randomized, multicenter phase III CASPIAN study demonstrated that first-line treatment with the PD-L1 inhibitor durvalumab plus EP gave rise to a significant OS improvement compared to EP alone (HR, 0.73; p = 0.0047) [5]. In the control arm, PCI was optional. Durvalumab plus EP was approved for ES-SCLC by the US authorities in March 2020 and is under review by other health authorities globally.
The CASPIAN study contained another experimental arm assessing the CTLA-4 inhibitor tremelimumab in addition to durvalumab and EP 3-weekly for 4 cycles followed by durvalumab maintenance. At the ASCO Congress, Paz-Ares et al. presented the primary analysis for the comparison between this group and the EP-only control patients [7]. According to this, the addition of tremelimumab to durvalumab and EP did not significantly improve OS over EP alone (10.4 vs. 10.5 months; HR, 0.82). Moreover, the investigators reported the results of planned updated analyses for durvalumab plus EP vs. EP. After an additional follow-up of 11 months, durvalumab in combination with chemotherapy continued to demonstrate OS improvement compared to a robust control arm that allowed up to 6 cycles and EP and the use of PCI (12.9 vs. 10.5 months; HR, 0.75; nominal p = 0.0032). The OS curves showed a sustained separation, with 22. % vs. 14.4 % of patients alive at 24 months. Durvalumab-related benefits were observed across all pre-specified subgroups and key secondary efficacy outcomes including PFS (24-month rates, 11.0 % vs. 2.9 %), objective response rates (67.9 % vs. 58.0 %; OR, 1.53) and duration of response (24-month rates, 13.5 % vs. 3.9 %). The safety findings in all arms remained consistent with the known safety profiles of all agents. These results further support the administration of durvalumab plus EP as a new standard-of-care treatment for first-line ES-SCLC, offering the flexibility of platinum choice (cisplatin vs. carboplatin).
KEYNOTE-604: pembrolizumab plus chemotherapy
Pembrolizumab has been approved in the third or later lines for patients with metastatic SCLC in several countries based on the KEYNOTE-028 and KEYNOTE-158 studies [8]. The randomized, placebo-controlled KEYNOTE-604 trial assessed pembrolizumab plus EP for four 3-weekly cycles in 228 treatment-naïve patients with stage IV SCLC [9]. This regimen was followed by pembrolizumab maintenance for up to 31 cycles. In the control group (n = 225), patients received EP plus placebo followed by placebo maintenance. Unstable brain metastases were not allowed.
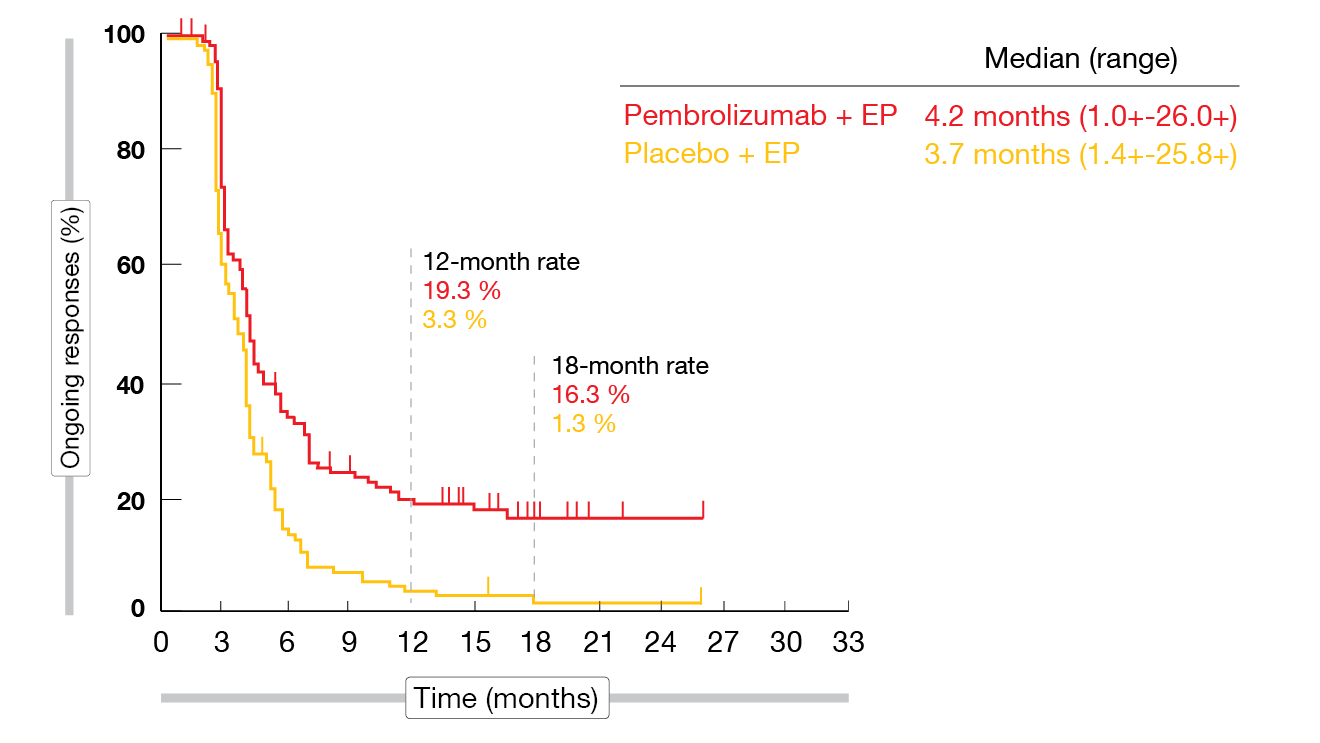
The addition of pembrolizumab to EP as first-line therapy significantly improved PFS compared to EP alone at the time of the second interim analysis that provided the final PFS analysis per protocol (4.5 vs. 4.3 months; HR, 0.75; p = 0.0023). According to the final analysis of the trial, the 18-month PFS rates were 10.8 % vs. 2.1 % (HR, 0.73). OS findings in the ITT population showed a 20 % reduction in the mortality risk (10.8 vs. 9.7 months; HR, 0.80; p = 0.0164), although the significance threshold was missed (p = 0.0128). At 24 months, 22.5 % vs. 11.2 % of patients were alive. Both PFS and OS subgroup analyses suggested improved results across subgroups in the experimental arm with the exception of patients with baseline brain metastases. The ORRs were 70.6 % vs. 61.8 % for pembrolizumab plus EP and EP, respectively. Complete responses resulted in 1.8 % vs. 0.9 %. Responses appeared to be durable in a subset of pembrolizumab-treated participants, with 18-month rates of 16.3 % vs. 1.3 % (Figure 2).
AEs of the combination were as expected and manageable. The rates for any-grade immune-mediated AEs were 24.7 % vs. 10.3 % in the as-treated population, with 5.8 % vs. 0.9 % leading to discontinuation. According to the authors, these data support the benefit of pembrolizumab and the value of immunotherapy in the treatment of SCLC.
Figure 2: Duration of response for pembrolizumab plus chemotherapy vs. placebo plus chemotherapy in the KEYNOTE-604 trial
Evaluation of nivolumab in ECOG-ACRIN EA5161
The ECOG-ACRIN EA5161 trial was conducted to assess the role of nivolumab in ES-SCLC [10]. Patients who had not received prior chemotherapy were randomized to EP plus nivolumab followed by nivolumab maintenance (n = 75) or chemotherapy only followed by observation (n = 70). The inclusion of patients with treated brain metastases was allowed.
For PFS, which was defined as the primary endpoint, the nivolumab-based regimen showed superiority with a median PFS of 5.5 vs. 4.7 months (HR, 0.68; p = 0.047). OS, as a secondary endpoint, was also in favor of the experimental arm, although not significantly so (11.3 vs. 9.3 months; HR, 0.73; p = 0.14). Objective responses resulted in 52 % vs. 47 %, with a median duration of response of 5.6 vs. 3.3 months. The combination of nivolumab and chemotherapy was well tolerated, and toxicities were manageable. Grade 3/4 AEs occurred across the treatment arms with similar frequency. In their conclusion, the investigators noted that ECOG-ACRIN EA5161 confirms the efficacy of nivolumab in ES-SCLC.
REFERENCES
- Turrisi AT et al., Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999; 340(4): 265-271
- Kubota K et al., Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): a randomised phase 3 study. Lancet 2014; 15(1): 106-113
- Hallqvist A et al., Accelerated hyperfractionated radiotherapy and concomitant chemotherapy in small cell lung cancer limited-disease. Dose response, feasibility and outcome for patients treated in western Sweden, 1998-2004. Acta Oncol 2007; 46(7): 969-974
- Grønberg BH et al., Randomized phase II study comparing the efficacy of standard-dose with high-dose twice-daily thoracic radiotherapy in limited stage small-cell lung cancer. J Clin Oncol 38: 2020 (suppl; abstr 9007)
- Paz-Ares L et al., Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019; 394: 1929-1939
- Horn L et al., First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379: 2220-2229
- Paz-Ares L et al., Durvalumab ± tremelimumab + platinum-etoposide in first-line extensive-stage SCLC: updated results from the phase 3 CASPIAN study. J Clin Oncol 38: 2020 (suppl; abstr 9002)
- Chung JC et al., Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol 2020; 15: 618-627
- Rudin CM et al., KEYNOTE-604: pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer. J Clin Oncol 38: 2020 (suppl; abstr 9001)
- Leal TA et al., Randomized phase II clinical trial of cisplatin/carboplatin and etoposide alone or in combination with nivolumab as frontline therapy for extensive stage small cell lung cancer: ECOG-ACRIN EA5161. J Clin Oncol 38: 2020 (suppl; abstr 9000)