Early-stage lung cancer: immunotherapeutic standards
IMpower010: adjuvant administration of atezolizumab
Despite established strategies such as platinum-based chemotherapy and EGFR-targeted agents, there is a high unmet need for improved adjuvant treatment in the setting of completely resected early-stage NSCLC (stage IB-IIIA). Therefore, the global phase III IMpower010 trial tested the anti-PD-L1 antibody atezolizumab 1,200 mg every 21 days for 16 cycles compared to best supportive care (BSC) in patients with stage IB-IIIA lung cancer who had undergone lobectomy or pneumonectomy followed by 1-4 cycles of chemotherapy. EGFR mutations and ALK rearrangements did not represent exclusion criteria in this study. Disease-free survival (DFS) was defined as the primary endpoint. This was tested hierarchically in three primary analysis populations: the PD-L1 tumor cell (TC) ≥ 1 % stage II-IIIA population (n = 476); the all-randomized stage II-IIIA population (n = 882); and the ITT population (stage IB-IIIA; n = 1,005).
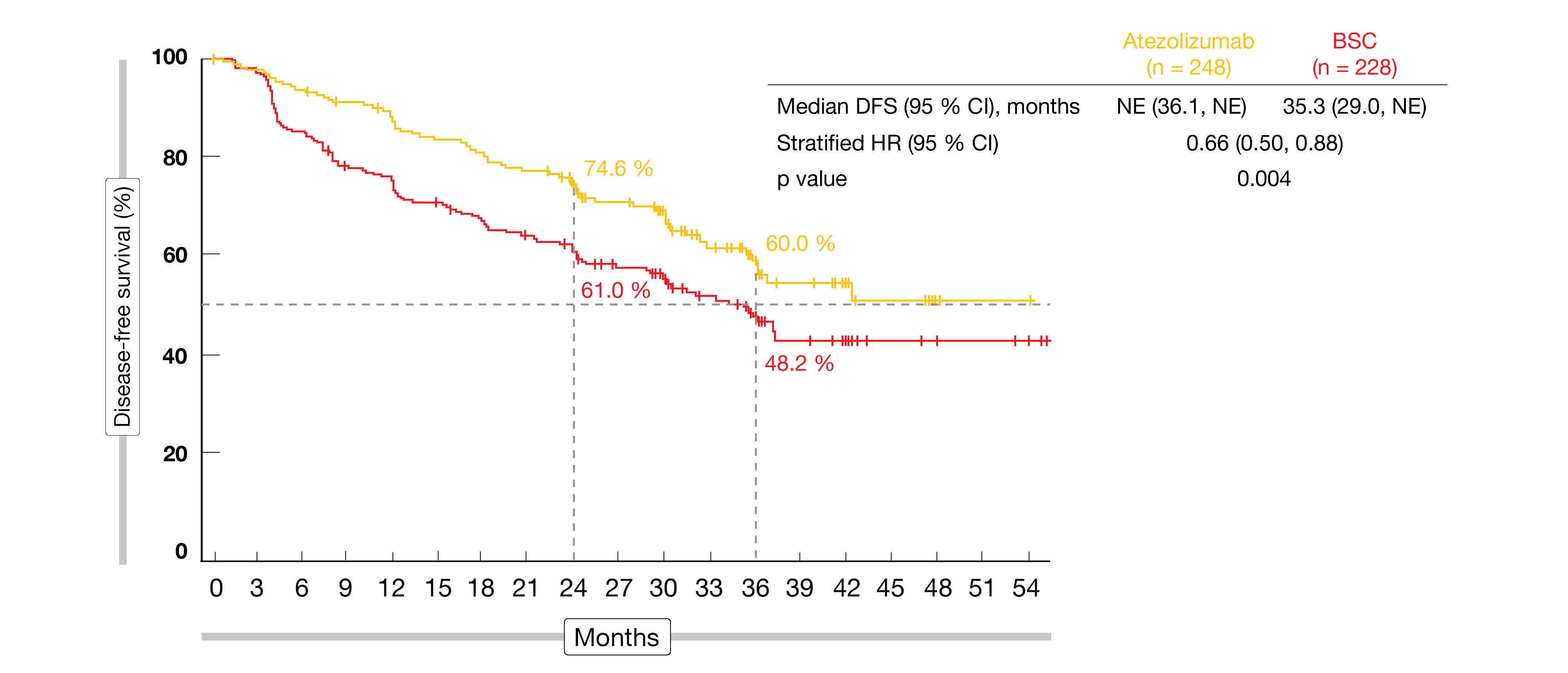
According to the pre-planned interim analysis presented by Wakelee et al. at the ASCO 2021 Annual Meeting, atezolizumab gave rise to a significant DFS benefit in both the PD-L1 TC ≥ 1 % stage II-IIIA population (not reached vs. 35.3 months; HR, 0.66; p = 0.004; Figure 1) and the all-randomized stage II-IIIA population (42.3 vs. 35.3 months; HR, 0.79; p = 0.02) [1]. The curves separated early and remained separated in both populations. Subgroup analyses of the all-randomized cohort indicated that the DFS benefit increased with PD-L1 expression, as risk reductions for the groups with PD-L1 TC ≥ 50 %, ≥ 1, and < 1 % were 57 %, 34 % and 3 %, respectively. In the ITT population including patients with stage IB disease, DFS did not cross the significance boundary at the time of the analysis (not reached vs. 37.2 months; HR, 0.81). Testing will continue in this group.
Overall survival (OS) data were immature and not formally assessed according to the statistical plan. However, a trend towards OS improvement emerged in the PD-L1 ≥ 1 % stage II-IIIA population (HR, 0.77). The safety profile of atezolizumab was consistent with prior experience with this treatment as single agent across indications and lines of therapy. Overall, IMpower010 is the first phase III study of cancer immunotherapy to demonstrate DFS improvement in the adjuvant NSCLC setting after platinum-based chemotherapy. The authors concluded that atezolizumab can be considered a practice-changing adjuvant treatment option for patients with PD-L1 TC ≥ 1 % stage II-IIIA non-small-cell lung tumors.
Figure 1: Superiority of atezolizumab vs. BSC for disease-free survival in the PD-L1 tumor cell ≥ 1 % stage II-IIIA population
Addition of neoadjuvant nivolumab
The randomized, phase III CheckMate 816 trial tested neoadjuvant use of nivolumab plus chemotherapy against chemotherapy alone in patients with newly diagnosed, resectable, stage IB-IIIA NSCLC. Forde et al. demonstrated that the combination yielded significant improvement in the primary endpoint of pathological complete response (pCR) while maintaining a tolerable safety profile [2]. At ASCO 2021, additional efficacy data and key surgical outcomes were reported [3].
Among the 179 patients randomized into each arm, a numerically greater proportion of those treated with nivolumab had definitive surgery (83 % vs. 75 %); in this group, fewer patients underwent pneumonectomy, and minimally invasive surgery was used more often. Baseline stage of disease did not affect pCR improvement. In stage IB/II, the median residual viable tumor percentages were 28 % vs. 79 % for nivolumab plus chemotherapy vs. chemotherapy; for stage IIIA, this was 8 % vs. 70 %. No differences occurred with respect to completeness of resection, although there was a numerical advantage in the experimental arm regarding R0 resections.
The neoadjuvant nivolumab plus chemotherapy regimen proved tolerable, and the addition of the PD-1 inhibitor did not increase the rate of post-surgical complications. Any-grade surgery-related adverse events were observed in 41 % vs. 47 %. Overall, the safety and surgical outcome data reported thus far from CheckMate 816, along with significant improvement in pCR, support the combination of nivolumab and chemotherapy as a potential neoadjuvant option for patients with resectable NSCLC. The study continues to mature for the other primary endpoint of event-free survival and further outcomes.
Gefitinib vs. chemotherapy in EGFR-mutant NSCLC
Although adjuvant cisplatin-based chemotherapy is a standard of care for patients with stage II-III, completely resected NSCLC, relapses are frequent. The randomized, phase III IMPACT trial conducted in Japan tested the assumption that adjuvant EGFR-TKI treatment improves the outcomes of patients with EGFR-mutated tumors [4]. In this study, patients after complete resection of stage II-III tumors were randomized to either gefitinib 250 mg/d for 24 months or cisplatin plus vinorelbine every 3 weeks for 4 cycles. Each arm included 116 individuals.
IMPACT did not meet its primary endpoint, as DFS was not significantly prolonged with gefitinib compared to chemotherapy (35.9 vs. 25.0 months; HR, 0.92; p = 0.63). At 5 years, 31.8 % vs. 34.1 % of patients were disease-free. However, according to the exploratory subgroup analysis, some patients such as those aged ≥ 70 years benefited from gefitinib. The OS analysis showed no difference, with almost superimposable curves. Again, the subgroup analysis demonstrated a benefit of the EGFR inhibition in the group aged ≥ 70 years.
Adjuvant gefitinib had acceptable toxicity. While grade 3/4 neutropenia and leukopenia were frequent in the cisplatin/vinorelbine-treated arm, this was negligible in the gefitinib arm where transaminase elevations and rash were most prevalent. Three treatment-related deaths occurred in the cisplatin/vinorelbine group due to cerebral infarction, suicide, and pneumonia. As the authors noted in their conclusion, the apparent non-inferiority of adjuvant gefitinib regarding DFS and OS might justify its use in selected subsets of patients, especially those deemed unsuitable for adjuvant chemotherapy with cisplatin/vinorelbine.
Lasting benefits at 5 years: PACIFIC
The randomized, double-blind, placebo-controlled, phase III PACIFIC trial has transformed the treatment of patients with unresectable stage III NSCLC whose disease has not progressed after platinum-based chemoradiation. In this setting, the anti-PD-L1 antibody durvalumab, when compared to placebo, significantly improved OS (p = 0.00251) and PFS (p < 0.0001) and was therefore established as a standard of care [5, 6]. Exploratory survival analyses were conducted approximately 5 years after the last patient had been randomized [7].
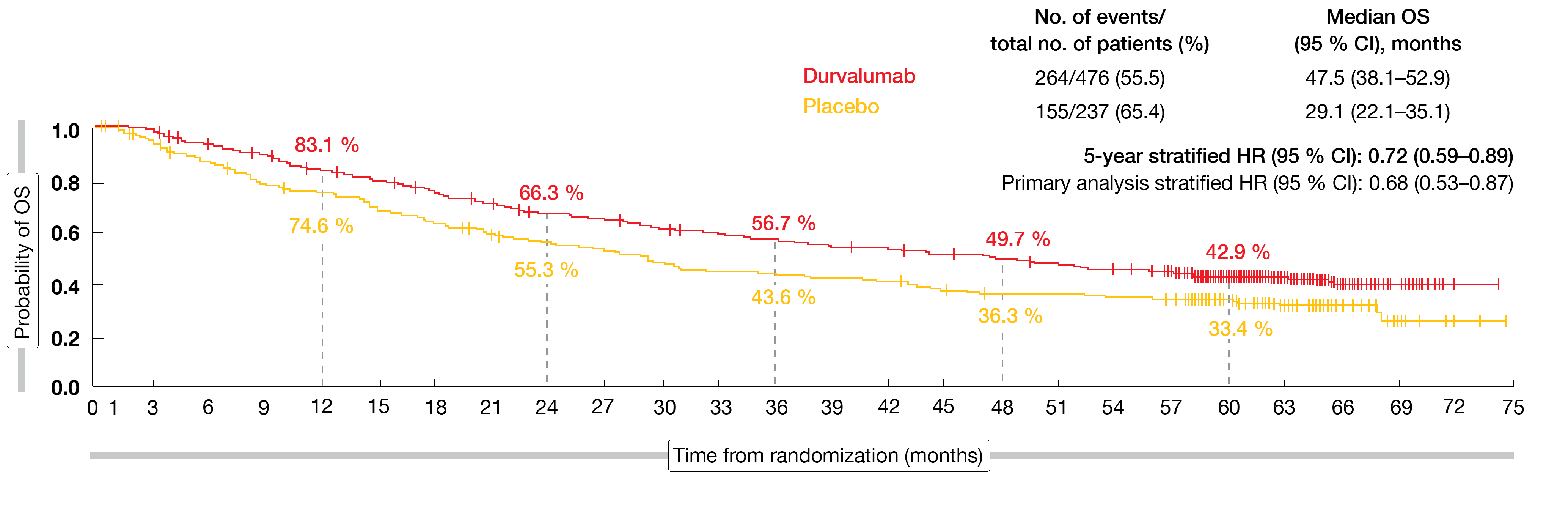
At that time, the median follow-up in all randomized patients was 34.2 months. The data showed that the OS and PFS benefits with durvalumab vs. placebo were consistent with the primary analyses [5, 6]. At 5 years, patients in the experimental arm still experienced a 28 % mortality risk reduction, with OS rates of 42.9 % vs. 33.4 % (Figure 2). For PFS, the 5-year rates were 33.1 % vs. 19.0 %, which translated into a 45 % reduction in the risk of progression or death. Likewise, updated OS and PFS for the subgroups were consistent with the results reported at the time of the primary analyses.
The authors noted that these findings demonstrate robust and sustained OS benefit and durable PFS benefit with the PACIFIC regimen. Approximately one third of the durvalumab-treated patients remained alive and free of disease progression at 5 years, which establishes a new benchmark for the standard of care in this setting.
Figure 2: Long-term overall survival benefit with durvalumab vs. placebo in the PACIFIC trial
ctDNA as predictor of early relapse
Liquid biopsies based on circulating tumor DNA (ctDNA) analysis are being investigated with the aim of detecting residual disease and recurrence in patients with localized NSCLC. The assessment of minimal residual disease might help to identify patients who may benefit from adjuvant therapy. Therefore, Gale et al. evaluated ctDNA in serial plasma samples using a personalized sequencing assay to explore the feasibility and prognostic value of ctDNA detection at or before relapse in stage IA-IIIB NSCLC patients after treatment with curative intent [8]. Eighty-eight individuals were included; 78.4 % of them underwent surgery, and 21.6 % received chemoradiation. Tumor exome sequencing was performed to identify somatic mutations, and a personalized ctDNA assay was developed for each patient. Plasma samples were collected before and after treatment, and at 3, 6, and 9 months. For 17 patients, additional plasma was collected at the time of disease progression. The patients were followed for a median of 3 years.
According to the findings, residual ctDNA predicts early relapse. Monitoring ctDNA at or prior to relapse using a sensitive patient-specific plasma sequencing assay proved feasible. ctDNA detection 2 weeks to 4 months after the end of treatment was associated with shorter relapse-free survival (HR, 14.8; p < 10-5) and OS (HR, 5.48; p < 0.0003). In patients who progressed, detection of ctDNA preceded clinical progression by a median lead time of 212.5 days.
Overall, these results support emerging evidence that ctDNA monitoring can reliably detect residual disease after treatment with curative intent many months before clinical progression and offers an opportunity to identify patients who might benefit from adjuvant therapy.
REFERENCES
- Wakelee HA et al., IMpower010: primary results of a phase 3 global study of atezolizumab vs best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer. J Clin Oncol 39, 2021 (suppl 15; abstr 8500)
- Forde PM et al., Nivolumab + platinum-doublet chemotherapy vs chemo as neoadjuvant treatment for resectable (IB-IIIA) non-small cell lung cancer in the phase 3 CheckMate 816 trial. AACR Annual Meeting 2021, abstract CT003
- Spicer J et al., Surgical outcomes from the phase 3 CheckMate 816 trial: nivolumab + platinum-doublet chemotherapy vs. chemotherapy alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer. J Clin Oncol 39, 2021 (suppl 15; abstr 8503)
- Tada H et al., Adjuvant gefitinib versus cisplatin/vinorelbine in Japanese patients with completely resected, EGFR-mutated, stage II-III non-small cell lung cancer (IMPACT. WJOG6410L): a randomized phase 3 trial. J Clin Oncol 39, 2021 (suppl 15; abstr 8501)
- Antonia SJ et al., Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377(20): 1919-1929
- Antonia SJ et al., Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379(24): 2342-2350
- Spiegel DR et al., Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC – an update from the PACIFIC trial. J Clin Oncol 39, 2021 (suppl 15; abstr 8511)
- Gale D et al., Residual ctDNA after treatment predicts early relapse in patients with early-stage NSCLC. J Clin Oncol 39, 2021 (suppl 15; abstr 8517)
© 2021 Springer-Verlag GmbH, Impressum