Enhancing immunosupportive mechanisms via anti-angiogenesis
Treatment with anti-angiogenic agents offers potential in the management of patients progressing on immune checkpoint inhibitors as it has been shown that excessive VEGF production can create an immunosuppressive tumor microenvironment by modulation of immune cell function and reduction of immune cell access [1-3]. This might contribute to checkpoint inhibitor resistance and prime the tumor for anti-angiogenic therapy.
VARGADO: nintedanib after chemo-immunotherapy
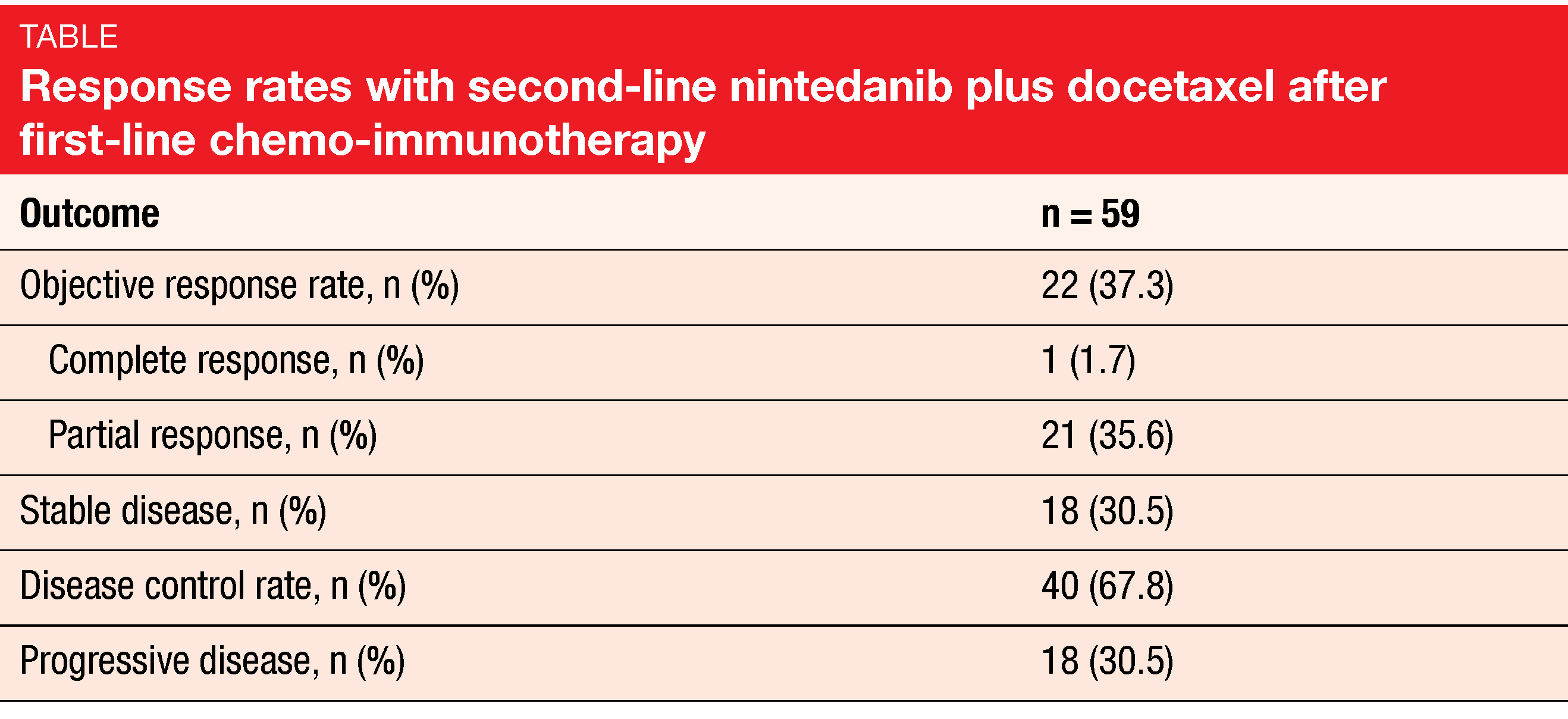
Anti-angiogenic treatment involving inhibition of VEGF, PDGF and FGF is assumed to enhance vessel normalization and to improve cell access to the tumor, thus favoring the restoration of an immunosupportive tumor microenvironment in the so-called angio-immunogenic switch [2]. The combination of docetaxel with the oral, triple angiokinase inhibitor nintedanib that targets VEGFR 1-3, PDGFR α/β, FGFR 1-3, and RET has been approved in many countries for the treatment of locally advanced, metastatic or locally recurrent adenocarcinoma of the lung after first-line chemotherapy. In the ongoing, prospective, non-interventional VARGADO study, nintedanib plus docetaxel is assessed under real-world conditions such as progression after chemotherapy and immunotherapy, to help inform clinical decision making. At ASCO 2021, Grohé et al. reported the initial efficacy data from Cohort C of the study that received second-line nintedanib plus docetaxel after progression on first-line chemo-immunotherapy [4]. The analysis included the first 100 patients treated in this cohort. In two thirds of cases, time since the start of first-line treatment was shorter than 9 months. Nintedanib plus docetaxel was administered according to the approved label.
After a median follow-up of 5.3 months, median PFS was 4.4 months, the overall response rate was 37.3 %, and disease control had been achieved in 67.8 % (Table). Overall survival data were still immature. Drug-related AEs mainly comprised diarrhea, nausea, and fatigue. Grade ≥ 3 treatment-emergent AEs occurred in 47 %. Thirty-one percent of patients had at least on nintedanib dose reduction, and in 16 %, at least one docetaxel dose reduction was performed. Investigator-defined drug-related treatment-emergent AEs led to study drug discontinuation in 16 %. No new safety signals or unexpected toxicities emerged.
In their conclusion, the authors noted that the initial data from Cohort C of the VARGADO study provide the first evidence that second-line nintedanib plus docetaxel has clinically meaningful efficacy and a manageable safety profile following progression on first-line chemo-immunotherapy. Recruitment and follow-up are ongoing in this cohort.
Combined VEGF/Ang2 and PD-1 inhibition
Another approach is the combination of anti-angiogenic and immunotherapeutic agents, such as the bispecific nanobody® BI 836880 that targets VEGF as well as Ang2 and the anti-PD-1 antibody ezabenlimab. BI 836880 antagonizes the immunosuppressive effects of VEGF and Ang2, thus reprogramming the tumor microenvironment [2, 5-7]. The addition of a PD-1 inhibitor drives T-cell-mediated tumor cell death. Both agents have demonstrated safety and preliminary anti-tumor activity as monotherapies in phase I studies [8, 9].
An ongoing phase Ib study aims to assess the safety and anti-tumor activity of the combination in patients with advanced or metastatic solid tumors. In the dose-escalation part, the recommended dose was defined as BI 836880 720 mg plus ezabenlimab 240 mg i. v. every 3 weeks. The cohort expansion part of the study includes seven cohorts with metastatic NSCLC, SCLC, glioblastoma, metastatic melanoma, and hepatocellular carcinoma. Cohorts A and B encompass NSCLC patients pretreated with immune checkpoint inhibitors and chemotherapy plus checkpoint inhibitors, respectively (n = 40 each). Cohort C contains SCLC patients after chemotherapy with or without immunotherapy (n = 30). Overall, 215 patients were included in the analysis presented at ASCO 2021 [10].
The combination gave rise to an overall response rate of 13 % in the total population, with 4 and 5 patients in Cohorts A and C, respectively, experiencing partial response. Stable disease was achieved in 61 % overall. All-grade treatment-related AEs emerged in 55 %, with asthenia (22 %), hypertension (19 %) and diarrhea (14 %) occurring most frequently. Immune-related AEs were observed in 16 %. The authors concluded that BI 836880 plus ezabenlimab shows preliminary antitumor activity and a manageable safety profile in a range of tumor types.
REFERENCES
- Popat S et al., Anti-angiogenic agents in the age of resistance to immune checkpoint inhibitors: Do they have a role in non-oncogene-addicted non-small cell lung cancer? Lung Cancer 2020; 144: 76-84
- Fukumura D et al., Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018; 15(5): 325-340
- van der Woude LL et al., Migrating into the tumor: a roadmap for T cells. Trends Cancer 2017; 3(11): 797-808
- Grohé C et al., Second-line nintedanib + docetaxel for patients with lung adenocarcinoma after failure on first-line immune checkpoint inhibitor combination therapy: initial efficacy and safety results from VARGADO Cohort C. J Clin Oncol 39, 2021 (suppl 15; abstr 9033)
- Hofmann I et al., Dual targeting of angiogenesis pathways: combined blockade of VEGF and Ang2 signaling. 8th Euro Global Summit on Cancer Therapy 2015
- Gerald D et al., Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res 2013; 73(6): 1649-1657
- Huang H et al., Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer 2010; 10(8): 575-585
- Le Tourneau C et al., First-in-human phase I trial of BI 836880, a VEGF/angiopoietin-2-blocking nanobody, given every 3 weeks in patients with advanced/metastatic solid tumors. J Clin Oncol 2018; 36(15_suppl): 12024
- Johnson ML et al., Phase I trial of the programmed death receptor 1 (PD-1) inhibitor, BI 754091, in patients (pts) with advanced solid tumors. J Clin Oncol 36, 2018 (suppl 5S; abstr 212)
- Girard N et al., Phase Ib study of BI 836880 (VEGF/Ang2 nanobody®) plus ezabenlimab (BI 754091; anti-PD-1 antibody) in patients with solid tumors. J Clin Oncol 39, 2021 (suppl 15; abstr 2579)
© 2021 Springer-Verlag GmbH, Impressum