KRAS, MET, ROS1, HER2: current perspectives
CodeBreaK100: sotorasib
Approximately 13 % of patients with adenocarcinoma of the lung harbor the KRASG12C mutation [1]. To date, no agent targeting this oncogenic driver has been licensed, although there is a need to improve outcomes in this population after progression on first-line treatment encompassing immune checkpoint inhibitors. The first-in-class, irreversible, selective KRASG12C inhibitor sotorasib has demonstrated durable clinical benefit in pretreated patients with KRASG12C-mutated, locally advanced or metastatic NSCLC in the single-arm phase II CodeBreaK100 trial [2]. In this study, 126 patients received sotorasib at a daily oral dose of 960 mg. Eighty-one percent of the participants were previously treated with both platinum-based chemotherapy and immunotherapy. At ASCO 2021, Skoulidis et al. presented updated efficacy and safety data including mature overall survival after a median follow-up of 15.3 months and reported outcomes across various patient subgroups [3].
Sotorasib continued to provide durable clinical benefit with median OS of 12.5 months and median PFS of 6.8 months. Overall, 37.1 % of patients responded, with 4 patients (3.2 %) achieving complete responses. Disease control was obtained in 80.6 %, and median duration of response was 11.1 months. Treatment-related AEs (TRAEs) were mostly grade 1/2 and proved generally manageable. Grade 3 TRAEs occurred in 19.8 % of patients. Diarrhea, nausea and elevated transaminases were observed most commonly. Dose modifications and discontinuations resulted in 22.2 % and 7.1 %, respectively.
The treatment with sotorasib exhibited broad and consistent clinical activity across a range of patient subgroups. ORR and median OS were favorable irrespective of baseline characteristics including age, number of prior lines of therapy, and type of pretreatment. Notably, in 13 patients after immune checkpoint inhibition who had not been exposed to platinum-based chemotherapy, sotorasib yielded an ORR of 69.2 %, with median OS of 17.7 months.
Improved activity in STK11-mutant disease
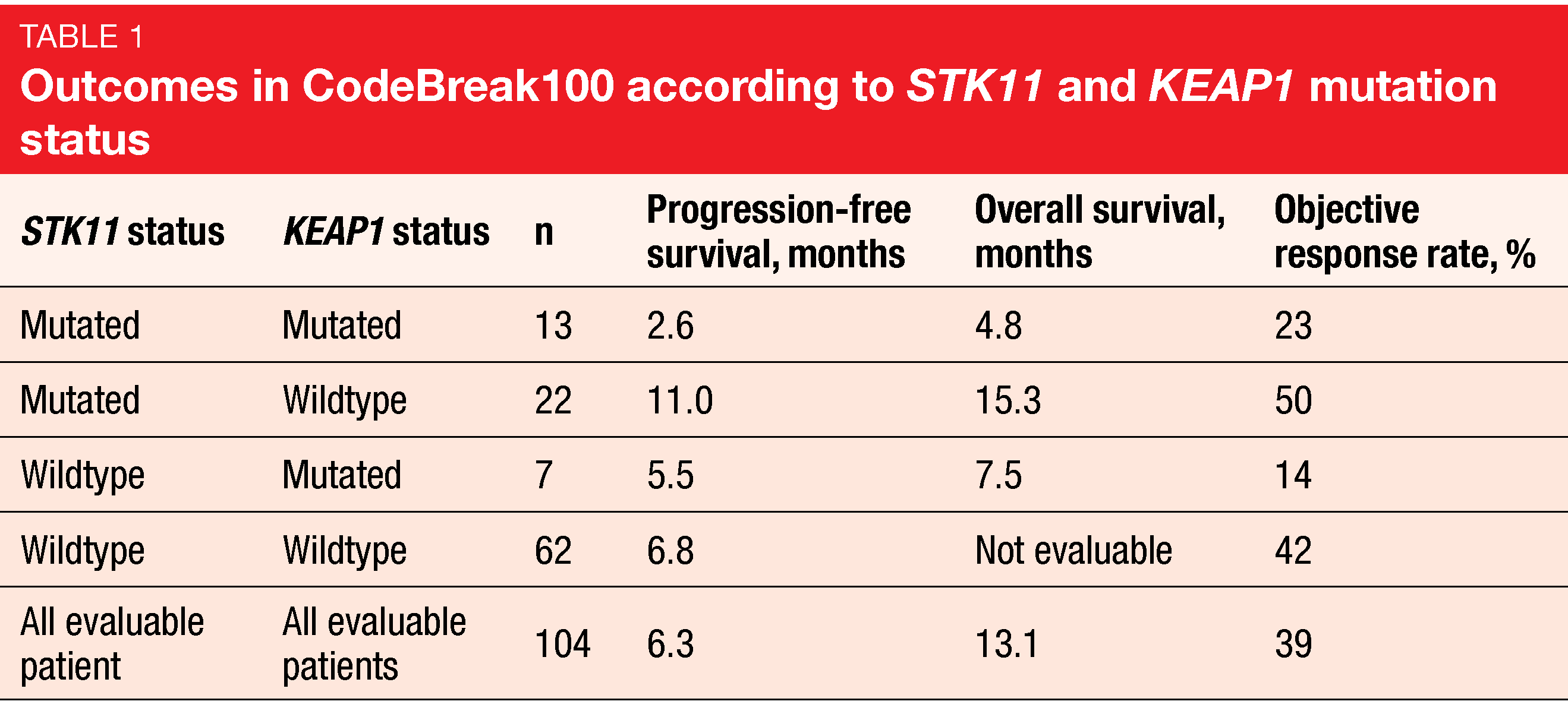
The prespecified exploratory analyses included the assessment of sotorasib in molecularly defined subgroups. These showed that the likelihood of achieving ORR was independent of KRASG12C mutant allele frequency. Moreover, ORRs did not vary across 84 patients with high or low tumor mutational burden (defined as ≥ 10 vs. < 10 mut/mb). Endpoints were also evaluated by co-occurring mutations in STK11 and KEAP1 (n = 104). This is of clinical relevance as inactivating somatic mutations in these genes have previously been linked to worse patient outcomes with standard-of-care therapies including chemotherapy and immunotherapy.
Here, improved efficacy of sotorasib treatment was seen in the STK11-mutant group with concurrent wild-type KEAP1, with an ORR of 50 %, which numerically exceeded the 39 % ORR in all evaluable patients. Median PFS in this group was 11.0 months compared to 6.8 months in patients with STK11 and KEAP1 wildtype and 6.3 months in the overall cohort (Table 1). Likewise, median OS was longest in the population harboring STK11 mutations and KEAP1 wildtype. KEAP1-mutant groups, on the other hand, appeared to derive less benefit from sotorasib treatment. However, these analyses are limited due to their exploratory nature and the small sample size. At present, the confirmatory phase III CodeBreaK200 trial evaluating sotorasib versus docetaxel in pretreated KRASG12C-mutated NSCLC is ongoing (NCT04303780).
Capmatinib: update of GEOMETRY mono-1
In the open-label, multi-cohort, phase II GEOMETRY mono-1 study, the oral, highly potent and selective MET inhibitor capmatinib has demonstrated clinically meaningful efficacy in patients with stage IIIB/IV NSCLC and MET exon 14 skipping mutations (METex14) [4]. Capmatinib has been approved in several countries for the treatment of patients with advanced METex14-positive NSCLC. GEOMETRY mono-1, which also enrolled patients with MET amplification, contains four METex14 cohorts: Cohort 5b and expansion Cohort 7 include treatment-naïve patients, while in Cohort 4 and expansion Cohort 6, pretreated patients are being evaluated. Overall, 160 patients were allocated to the four groups. At ASCO 2021, Wolf et al. reported preliminary data for Cohort 7 (n = 32) as well as other updated results [5].
According to the analysis, the ORR was 65.6 % in this group, which was in line with the previously reported ORR of 67.9 % for Cohort 5b [4]. Median PFS was 10.8 months in Cohort 7, while median OS had not been reached yet. Clinically meaningful OS results were obtained for treatment-naïve patients from Cohort 5b and pretreated patients from Cohort 4, in whom median OS was 20.8 and 13.6 months, respectively. The authors emphasized the long-term survival benefit conferred by capmatinib in these populations. Cohort 4 that contained patients treated in the second and third lines showed an ORR of 40.6 %. In Cohort 6, which was restricted to the second-line setting, ORR was 51.6 %. Responses occurred early on.
The manageable safety profile of capmatinib remained unchanged after the prolonged follow-up. Among treatment-related AEs, peripheral edema and nausea were most common (any grade, 46.1 % and 34.3 %, respectively). Four treatment-related fatal serious AEs occurred (i.e., cardiac arrest, hepatitis, organizing pneumonia, pneumonitis). In their conclusion, the authors noted that these updated results further confirm METex14 as a targetable oncogenic driver in NSCLC and strengthen the evidence for capmatinib as a valuable option in this setting.
Tepotinib for patients with MET amplification
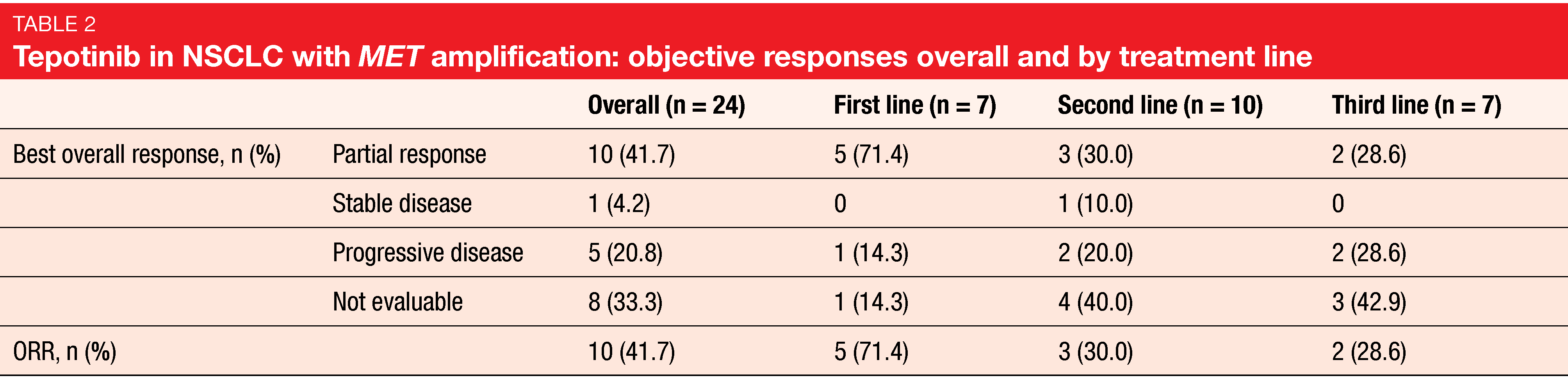
Lung cancer patients harboring MET amplification, which is present as an oncogenic driver in 1-5 % of NSCLC cases [6], have poor prognosis [7]. There is an urgent unmet need for new treatments in this population. The highly selective, oral, once daily MET inhibitor tepotinib has been approved for the treatment of metastatic NSCLC with METex14 in Japan and the US based on Cohort A of the open-label, multicenter, phase II VISION trial [8, 9]. At ASCO 2021, Le et al. reported the first data from Cohort B, which assessed tepotinib in patients with advanced NSCLC and MET amplification, as detected by liquid biopsy, in the absence of METex14 [10]. Patients enrolled in Cohort B had EGFR and ALK wildtype and were treated in the first, second or third line. Prior immunotherapy was allowed. Overall, 24 individuals received tepotinib 500 mg/d, which was predominantly administered in the second line. ORR by independent review committee was defined as the primary endpoint.
In this first study of a MET inhibitor in advanced NSCLC with MET amplification prospectively detected by liquid biopsy, tepotinib showed high and clinically meaningful activity. Overall, 41.7 % of patients responded to treatment. Patients receiving tepotinib in the first line appeared to be more sensitive to therapy. Subgroup analyses yielded response rates of 71.4 %, 30.0% and 28.6 % for the first, second, and third lines, respectively (Table 2). In the overall population, median PFS was 4.2 months, with a 9-month PFS rate of 40 %. In the first, second and third lines, the 9-month PFS rates were 51 %, 58 %, and not estimable. Median duration of response was still immature; at 9 months, 67 % of patients had ongoing responses.
Tepotinib was well tolerated, with mostly mild or moderate treatment-related AEs. Peripheral edema was the most common AE (any grade, 37.5 %), followed by generalized edema and constipation. Grade 3/4 treatment-related AEs occurred in 29.2 % but did not give rise to treatment discontinuation. According to the authors, tepotinib warrants further evaluation in patients with MET-amplified advanced NSCLC.
ROS1-positive NSCLC: activity of brigatinib
Crizotinib was the first agent to be approved for the treatment of patients with ROS1-fusion-positive NSCLC. However, no standard options have been introduced for crizotinib-resistant ROS1-positive disease to date. The single-arm, multicenter, phase II basket trial Barossa evaluated the second-generation ALK/ROS1 inhibitor brigatinib in advanced solid tumors with ROS1 fusion positivity. Daga et al. reported the results for Cohort 2 of the study that included 19 crizotinib-pretreated NSCLC patients from 9 institutions. ORR was defined as the primary endpoint [11].
In this group, brigatinib showed modest activity with an ORR of 26.3 % and a disease control rate of 57.9 %. Median PFS assessed by independent review and OS were 7.3 and 12.2 months, respectively. At 1 year, 57.4 % of patients were alive, and 26.9 % were progression-free. The safety profile of brigatinib including diarrhea, transaminase elevations and amylase elevations was consistent with previous studies. No grade 4/5 AEs occurred. Enrollment of the cohort 1 of the Barossa study that contains ROS1-inhibitor–naïve patients is ongoing.
HER2-targeted approach plus docetaxel
Approved therapies are lacking for NSCLC patients with HER2 aberrations that are oncogenic drivers in 1-2 % of cases [12]. The aim of the multicenter, single-arm, phase II IFCT-1703 R2D2 trial presented by Mazieres et al. was to prospectively evaluate a combination of two HER2-directed antibodies with docetaxel in this setting [13]. Forty-six pretreated patients with stage III/IV NSCLC and HER2 exon 20 insertion or mutation received pertuzumab 420 mg plus trastuzumab 6 mg/kg and docetaxel 75 mg/m2 from cycle 2 every 3 weeks.
Confirmed ORR, which was defined as the primary endpoint, was 28.9 % with this regimen. Stable disease resulted in 57.8 %. Median PFS and OS were 6.8 and 17.6 months, with 12-month rates of 29.0 % and 68.3 %, respectively. Treatment-related AEs mainly included diarrhea, fatigue, anemia, nausea, stomatitis, and decreased neutrophil counts. Among grade 3/4 AEs, decreased neutrophil counts occurred most frequently, followed by diarrhea. No pulmonary or cardiac toxicity was observed.
As the authors concluded, the triplet of trastuzumab, pertuzumab and docetaxel is feasible and active in pretreated, advanced, HER2-positive NSCLC. These results confirm the activity of HER2-antibody–based strategies which should be considered in these patients.
REFERENCES
- Biernacka A et al., The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet 2016; 209(5): 195-198
- Li BT et al., CodeBreaK 100: registrational phase 2 trial of sotorasib in KRAS p.G12C mutated non-small cell lung cancer. WCLC 2020, PS01.07
- Skoulidis F et al., Overall survival and exploratory subgroup analyses from the phase 2 CodeBreaK100 trial evaluating sotorasib in pretreated KRAS P.G12C mutated non-small cell lung cancer. J Clin Oncol 39, 2021 (suppl 15; abstr 9003)
- Wolf J et al., Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med 2020; 383(10): 944-957
- Wolf J et al., Capmatinib in MET exon 14-mutated, advanced NSCLC: updated results from the GEOMETRY mono-1 study. J Clin Oncol 39, 2021 (suppl 15; abstr 9020)
- Drilon A et al., Targeting MET in lung cancer: will expectations finally be MET? J Thorac Oncol 2017; 12(1):15-26
- Dimou A et al., MET gene copy number predicts worse overall survival in patients with non-small cell lung cancer (NSCLC); a systematic review and meta-analysis. PLos One 2014; 9(9): e107677
- Paik PK et al., Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med 2020; 383(10): 931-943
- Paik PK et al., Tepotinib in patients with MET exon 14 (METex14) skipping advanced NSCLC: updated efficacy from VISION Cohort A. J Thorac Oncol 2021; 16(3S): S174 (MA11.05)
- Le X et al., Tepotinib in patients with advanced non-small cell lung cancer with MET amplification. J Clin Oncol 39, 2021 (suppl 15; abstr 9021)
- Daga H et al., Phase II study of brigatinib in ROS1-positive non-small cell lung cancer patients previously treated with crizotinib: Barossa cohort 2. J Clin Oncol 39, 2021 (suppl 15; abstr 9040)
- Mazières J et al., Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016; 27(2): P281-286
- Mazières J et al., Combination of trastuzumab, pertuzumab and docetaxel in patients with advanced non-small cell lung cancer harboring HER2 mutation. Results from the IFCT-1703 R2D2 trial. J Clin Oncol 39, 2021 (suppl 15; abstr 9015)
© 2021 Springer-Verlag GmbH, Impressum