Opening up new vistas for patients with SCLC
Cisplatin vs. carboplatin in LS-SCLC
Concurrent chemo-radiation with a platinum-etoposide backbone constitutes the standard of care in limited-stage small-cell lung cancer (LS-SCLC). Here, cisplatin is traditionally the preferred platinum agent. However, data on the comparative efficacy of the less toxic carboplatin in this setting are lacking. To fill this gap, Azar et al. conducted a retrospective study based on the National VA Cancer Cube database [1]. Patients with pathologically confirmed LS-SCLC who were treated with concurrent chemo-radiation containing platinum-based multiagent chemotherapy were included. Overall, the analysis comprised 1,756 individuals. Among these, 801 and 1,018 received carboplatin and cisplatin, respectively, while 63 received both. Notably, patients aged ≥ 70 were more likely to be treated with carboplatin, while in the other age groups, cisplatin prevailed.
With respect to overall survival, the Kaplan-Meier curves for the two agents were shown to be superimposable. Median OS across stages I-III was 2.24 and 2.13 years for cisplatin and carboplatin, respectively (HR, 1.040; p = 0.462). The researchers also assessed OS according to several variables. No significant differences between cisplatin and carboplatin emerged for all ECOG performance status groups (0, 1, 2), with HRs of 1.066, 0.977, and 1.216, respectively. Of course, OS was generally shorter with decreasing performance status. This also applied to age; here, younger patients (50-59 and 60-69 years) had longer OS than those aged 70 years or older. Again, however, cisplatin and carboplatin performed equally well in all groups (HRs, 1.021, 0.944, and 1.020, respectively). Similarly, TNM stage (I, II, III) did not identify any patients with greater benefits from one treatment or the other (HRs, 1.221, 1.034, and 1.020, respectively).
Accounting for all variables, the multivariable analysis showed no significant differences between cisplatin and carboplatin. As the authors concluded, concurrent chemoradiation with carboplatin-etoposide confers similar OS compared to cisplatin-etoposide in patients with LS-SCLC irrespective of performance status and age. The favorable toxicity profile of carboplatin and comparable OS benefit identify it as an acceptable option in this setting.
Advanced disease: BiTE® therapy
The delta-like ligand 3 (DLL3) is a promising target in SCLC due to its high expression in tumor tissue and minimal expression in normal cells [2]. It has been validated as a therapeutic target in previous studies [3, 4]. The DLL3-targeting, half-life–extended bispecific T-cell engager (BiTE®) tarlatamab (AMG 757) engages the patient’s own T-cells to attack and eradicate DLL3-expressing cancer cells [5, 6]. At ASCO 2021, Owonikoko et al. presented updated safety, efficacy, and pharmacokinetic data from 66 patients included in the open-label, multicenter phase I study investigating tarlatamab in relapsed or refractory SCLC [7]. The study participants had received ≥ 1 line of systemic treatment and had progressed or recurred following ≥ 1 platinum-based chemotherapy.
The results support tarlatamab as the first half-life–extended BiTE® immune-oncology therapy in SCLC with an acceptable safety profile and encouraging efficacy across the dose range (i.e., 0.003-100 mg i. v. 2-weekly). Confirmed partial responses were observed in 20 % of patients, and the disease control rate was 47 %. For patients with confirmed PR, median responses lasted for a median of 8.7 months. Tarlatamab exhibited a manageable safety profile. Cytokine release syndrome (CRS) was the most common treatment-related AE (all grades, 44 %), followed by pyrexia (26 %) and fatigue (17 %). Grade ≥ 3 treatment-related AEs occurred in 27 %, which included only one CRS event (2 %). Treatment-emergent AEs resulted in discontinuation in 5 % of patients. Tarlatamab serum levels increased proportionally with the evaluated doses. Eight patients (14 %) developed treatment-emergent anti-tarlatamab binding antibodies, with no apparent impact on serum levels or AEs. The study is ongoing.
Multiomic characterization
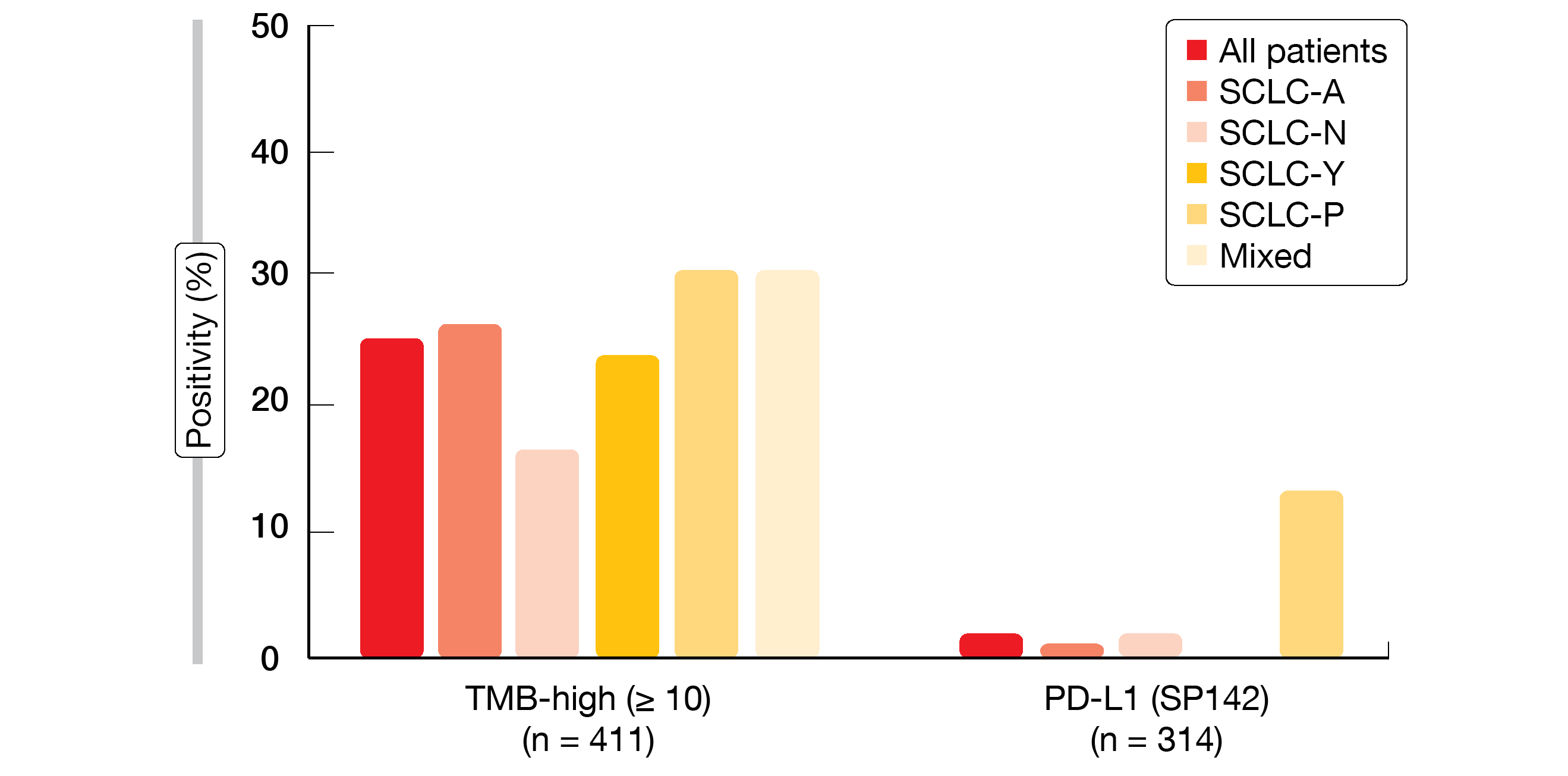
Based on the dominant expression of four lineage-defining transcription factors (ASCL1, NEUROD1, YAP1, POU2F3), SCLC has been divided into four subtypes (SCLC-A/N/Y/P) [8]. Puri et al. conducted comprehensive molecular profiling of 437 small-cell lung neuroendocrine tumors (including 7.3 % high-grade neuroendocrine lung carcinomas) using next-generation DNA sequencing (592-gene panel), RNA sequencing (whole transcriptome) and immunohistochemistry [9]. Tumors were stratified into five subgroups (SCLC-A/N/Y/P and mixed) based on the relative expression of the four transcription factors.
This analysis represents the largest real-world dataset of human SCLC tumors profiled by next generation DNA and whole transcriptome sequencing. It revealed differential expression of immune genes and predictive biomarkers across the subtypes. For instance, the SCLC-Y subtype showed the highest median expression of immune-related signatures and immune-related cell types. The highest expression of SLFN11 and SSTR2 genes was observed in the SCLC-N subtype, while MYC gene expression was highest in SCLC-P. This subtype also most frequently demonstrated high tumor mutational burden, along with the mixed group, and showed significantly increased PD-L1 expression according to the SP142 assay (13 %; p = 0.0046; Figure). CNS metastases mainly originated in the neuroendocrine-high subtypes (SCLC-A and SCLC-N). The RB1 mutation frequency was highest in the ASCL1 group (79.2 %) and lowest in the YAP1 group (49.4 %). According to the researchers, differential expression of genes and biomarkers might inform therapeutic vulnerabilities for rational and personalized treatment approaches in SCLC.
Figure: Clinically relevant biomarkers of response to immunotherapy across transcriptionally defined SCLC subtypes
REFERENCES
- Azar I et al., Cisplatin vs carboplatin for the treatment of limited-stage small cell lung cancer. J Clin Oncol 39, 2021 (suppl 15; abstr 8565)
- Leonetti A et al., Notch pathway in small-cell lung cancer: from preclinical evidence to therapeutic challenges. Cell Oncol (Dordr) 2019; 42(3): 261-273
- Rudin CM et al., Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017; 18(1): 42-51
- Morgensztern D et al., Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res 2019; 25(23): 6958-6966
- Stieglmaier J et al., Utilizing the BiTE (bispecific T-cell engager) platform for immunotherapy of cancer. Expert Opin Biol Ther 2015; 15(8): 1093-1099
- Einsele H et al., The BiTE (bispecific T-cell engager) platform: Development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 2020; 126(14): 3192-3201
- Owonikoko TK et al., Updated results from a phase 1 study of AMG 757 (tarlatamab), a half-life extended bispecific T-cell engager (HLE BiTE®) immune-oncology therapy targeting delta-like ligand 3 (DLL3), in small cell lung cancer. J Clin Oncol 39, 2021 (suppl 15; abstr 8510)
- Rudin CM et al., Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 2019; 19(5): 289-297
- Puri S et al., Real-world multiomic characterization of small cell lung cancer subtypes reveal differential expression of clinically relevant biomarkers. J Clin Oncol 39, 2021 (suppl 15; abstr 8508)
© 2021 Springer-Verlag GmbH, Impressum