Approaching marginal zone lymphoma from various angles
Approximately 10 % of Non-Hodgkin lymphomas are classified as marginal zone lymphoma (MZL) [1]. This is a heterogeneous malignancy with three main subtypes (i.e., extranodal, nodal, splenic) arising from memory B cells in the marginal zone of secondary lymphoid follicles [2, 3]. Due to its rarity and heterogeneous nature, the optimal therapeutic strategies for patients with MZL have been difficult to define. Advanced disease is largely considered incurable, with continuing patterns of relapse and remission. However, phase II data presented at the ASH 2020 Congress showed that emerging treatment options such as the BTK inhibitor zanubrutinib and the PI3Kδ inhibitor parsaclisib have the potential to change the course of disease in a considerable proportion of patients.
MAGNOLIA: patients with high-risk features
BTK inhibition has been hypothesized to work in MZL patients based on the observation that B-cell-receptor–mediated signaling is a critical step in the pathogenesis of this disease [4]. Indeed, the first-generation BTK inhibitor ibrutinib was shown to be active in the relapsed/refractory (r/r) setting [5] and has been granted accelerated approval by the FDA as monotherapy in patients pretreated with ≤ 1 anti-CD20-based regimen.
In an early-phase study, the next-generation BTK inhibitor zanubrutinib induced an 80 % ORR in 20 patients with r/r MZL [6]. Therefore, the multicenter, open-label, single-arm, phase II MAGNOLIA study investigated single-agent zanubrutinib 160 mg twice daily in 68 patients with r/r MZL who had received ≤ 1 prior line of CD20-directed therapy. ORR by independent review committee (IRC) using the Lugano classification was defined as the primary outcome. At ASH 2020, Opat et al. reported the response findings according to investigator assessment after a median follow-up of 10.7 months, while the blinded response assessment by IRC was ongoing [7].
MAGNOLIA generally enrolled patients with high-risk features. Sixty percent were aged ≥ 65 years, and 28 % were even 75 years or older. Two thirds and one third had relapsed and refractory disease, respectively. All subtypes of MZL were included, with 38.2 % showing the nodal subtype that conveys a poorer prognosis than the extranodal subtype. Lymphoma involvement in bone marrow was present in 42.6 %. A median of 2 lines of systemic therapy had been administered prior to study inclusion. At the time of the analysis, 44 patients remained on treatment.
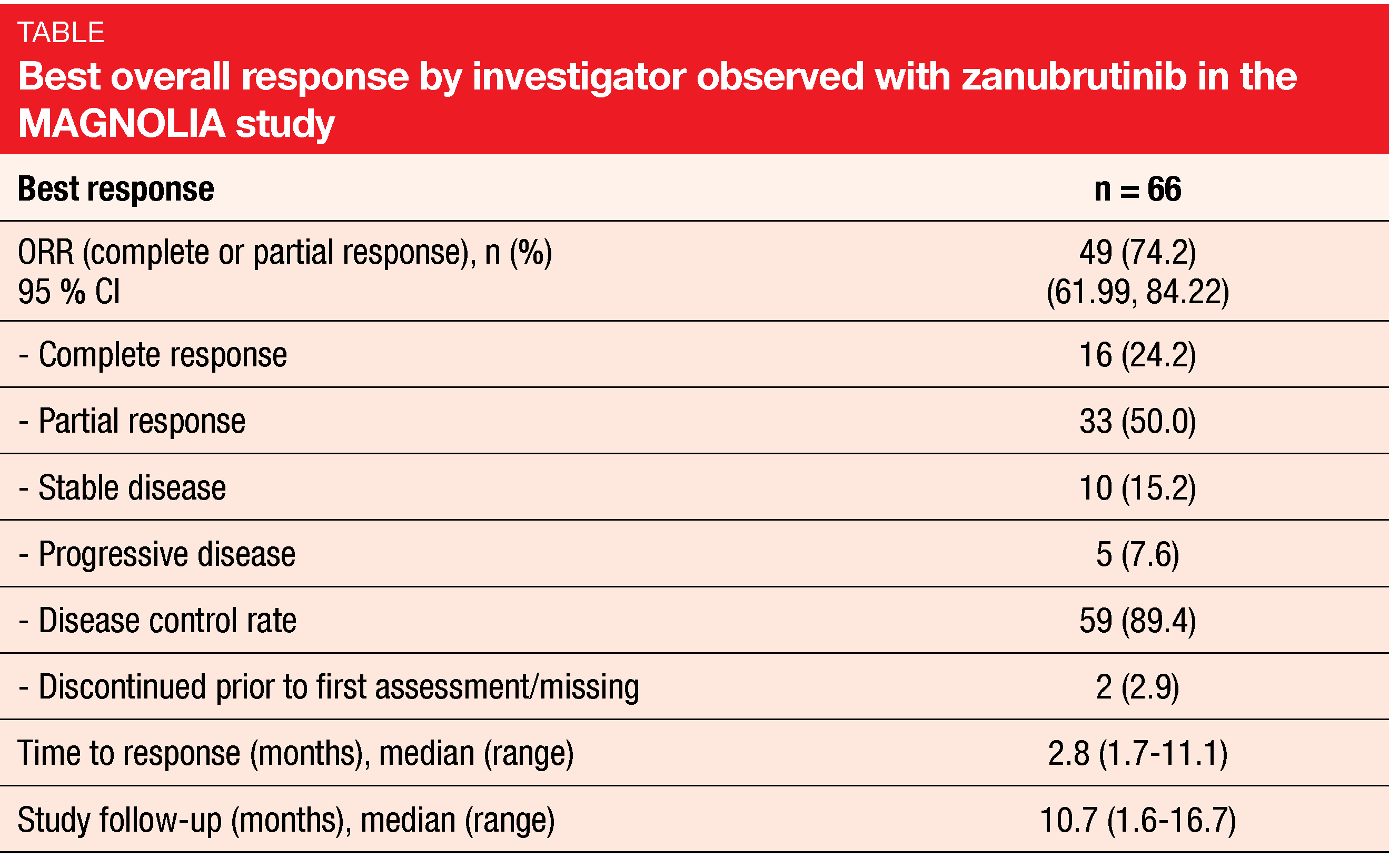
Clinical benefit of almost 90 %
Zanubrutinib proved to be highly active in this population. Overall, 74.2 % of patients responded, with complete remissions resulting in 24.2 % (Table). Clinical benefit (complete and partial responses plus stable disease) was observed in 89.4 %. Median time to response was short at 2.8 months. The majority of patients developed reductions in their tumor burden. Responses were generally consistent across subgroups with respect to MZL subtype, age, number of prior lines of systemic therapy and nature of prior treatment, among others. The ORRs were 89 % in patients aged ≥ 75 years, 65 % in those after ≥ 3 treatment lines, and 71 % in those with refractory disease. Almost 80 % of the entire group were still responding at 6 months.
Median PFS and OS had not been reached yet. At 9 months, 67 % of patients were progression-free, and at 12 months, 94 % were alive. Zanubrutinib showed a favorable AE profile and was generally well tolerated, which is also mirrored by the 99.6 % median relative dose intensity. Grade ≥ 3 treatment-emergent AEs (TEAEs) occurred in 38.2 %. In 23.5 %, dose interruptions were performed, while no patient required dose reductions. Only 2.9 % discontinued treatment due to TEAEs. Among TEAEs of interest, infection, hemorrhage, diarrhea and neutropenia prevailed. One patient each developed atrial fibrillation and atrial flutter. No major hemorrhages and no cases of hypertension were observed.
Preliminary results from CITADEL-204
The potent, highly selective, next-generation PI3Kδ inhibitor parsaclisib has shown promising clinical activity in r/r B-cell lymphomas including MZL in early trials [8]. Therefore, the open-label, phase II CITADEL-204 study assessed two parsaclisib treatment schedules in patients with r/r MZL who had received ≥ 1 prior systemic therapy including ≥ 1 anti-CD20 antibody as monotherapy or chemoimmunotherapy combination. The study was designed to include a BTK-inhibitor–naïve and an ibrutinib-experienced cohort. However, the latter was eventually terminated due to slower-than-expected enrollment.
Patients enrolled in the study were initially allocated to either parsaclisib 20 mg daily for 8 weeks followed by 20 mg once weekly (weekly group) or parsaclisib 20 mg daily for 8 weeks followed by 2.5 mg daily continuously (daily group). The daily group regimen was selected as the preferred regimen during the study, and all subsequent patients were enrolled into this group. ORR constituted the primary endpoint. At ASH 2020, Phillips et al. presented preliminary efficacy and safety data from the BTK inhibitor-naïve cohort for all treated patients (n = 100) and the daily group (n = 72) [9]. At data cutoff, the median duration of follow-up was 16.7 and 14.9 months, for all treated patients and the daily group, respectively.
Rapid and durable responses
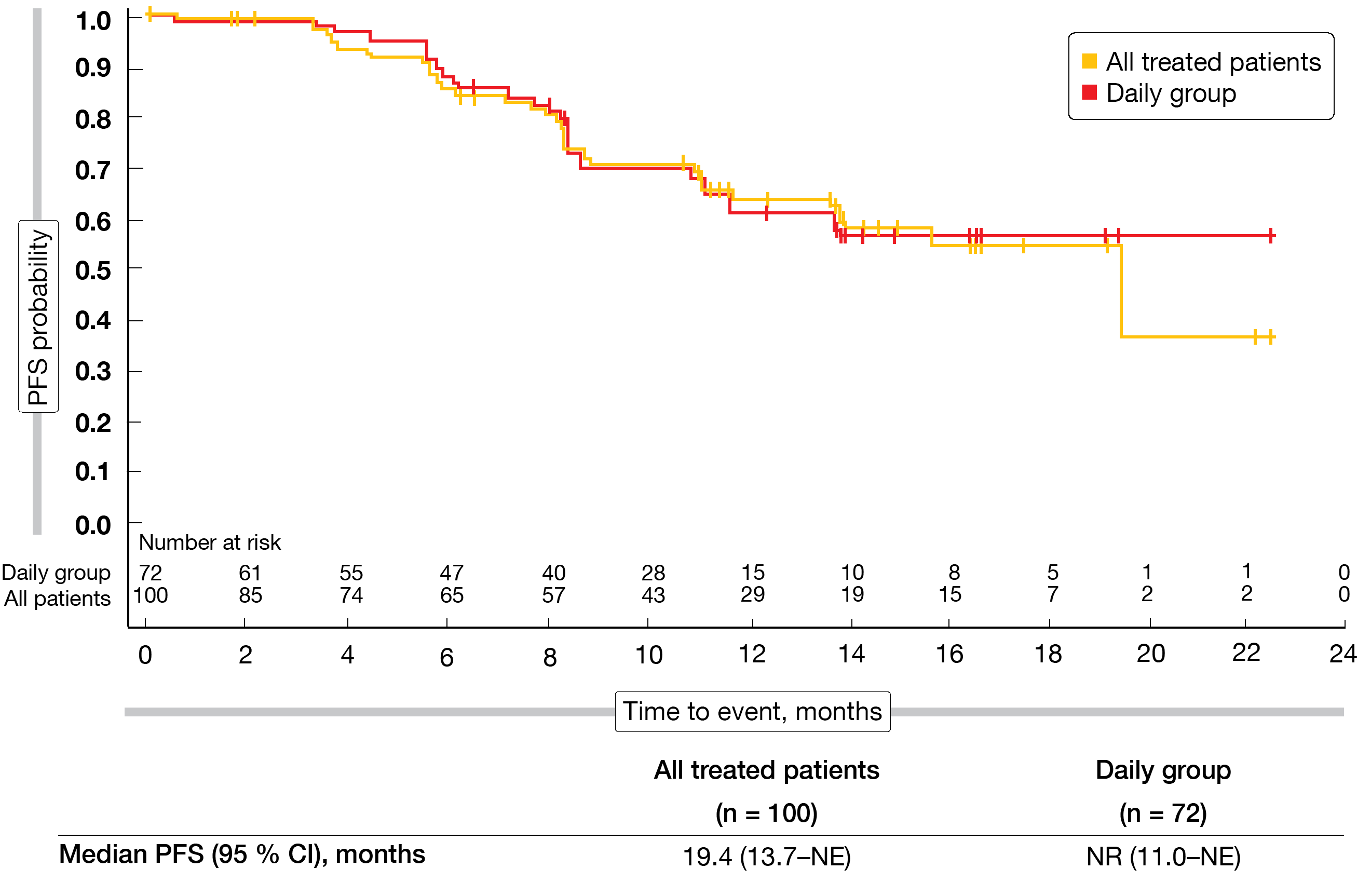
The ORR by independent review was 57.0 % and 56.9 % in all patients and the daily group, respectively. Complete remission resulted in 6.0 % and 5.6 %, respectively. ORRs did not differ significantly across patients with nodal, extranodal and splenic MZL; this also applied to the groups who were refractory to prior therapy and those who had relapsed on it. Sixty-seven percent of responders had either complete or partial responses already at the time of the first assessment. Median time to first response was 8.1 weeks. In the total group, median duration of response and median PFS were 12.0 months and 19.4 months (Figure), respectively. In the daily group, neither median duration of response nor median PFS (Figure) had been reached yet.
Parsaclisib showed a manageable safety profile. Diarrhea, cough and rash were observed as the most common AEs. The most common grade ≥ 3 AEs were diarrhea and neutropenia. Among serious TEAEs, pneumonia (7 %), colitis (5 %), diarrhea (5 %) and febrile neutropenia (5 %) showed the highest incidence. A total of 27 patients discontinued treatment due to TEAEs, with diarrhea or colitis events occurring in 14 individuals. The median time to onset of grade ≥ 3 diarrhea/colitis events was 5.3 months, and the median time to improvement to grade ≤ 2 was 12.0 days. In their conclusion, the authors noted that parsaclisib represents a potential new treatment option and first-in-class PI3Kδ inhibitor for MZL patients.
Figure: Progression-free survival in all patients treated with parsaclisib and in the cohort receiving continuous daily parsaclisib doses
REFERENCES
- Leslie LA et al., Contemporary management of nodal and primary splenic marginal zone lymphoma. Expert Rev Hematol 2019; 12(12): 1011-1022
- Denlinger NM et al., Management of relapsed/refractory marginal zone lymphoma: focus on ibrutinib. Cancer Manag Res 2018; 10: 615-624
- Kahl B, Yang D, Marginal zone lymphomas: management of nodal, splenic, and MALT NHL. Hematology Am Soc Hematol Educ Program 2008: 359-364
- Seiler T, Dreyling M, Bruton’s tyrosine kinase inhibitors in B-cell lymphoma: current experience and future perspectives. Expert Opin Investig Drugs 2017; 26(8): 909-915
- Noy A et al., Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 2017; 129(16): 2224-2232
- Tedeschi A et al., Phase 1/2 study of single-agent zanubrutinib in patients with relapsed/refractory marginal zone lymphoma. EHA 2020, abstract EP1165
- Opat S et al., Efficacy and safety of zanubrutinib in patients with relapsed/refractory marginal zone lymphoma: initial results of the MAGNOLIA (BGB-3111-214) trial. ASH 2020, abstract 339
- Forero-Torres A et al., Parsaclisib, a potent and highly selective PI3Kδ inhibitor, in patients with relapsed or refractory B-cell malignancies. Blood 2019; 133(16): 1742-1752
- Phillips T et al., Phase 2 study evaluating the efficacy and safety of parsaciclib in patients with relapsed or refractory marginal zone lymphoma (CITADEL-204). ASH 2020, abstract 338
© 2020 Springer-Verlag GmbH, Impressum