Insights from early clinical trials on targeted treatment in B-cell malignancies
DTRM-555: fixed-dose combination
Richter’s transformation (RT), which describes transformation of CLL/SLL to diffuse large B-cell lymphoma (DLBCL) or Hodgkin lymphoma, is a rare event occurring in approximately 5–7 % of CLL cases [1]. However, defined standards of care are lacking, and outcomes are generally poor once patients are refractory to rituximab plus chemotherapy. A new strategy is the synthetic lethality approach that describes simultaneous inhibition of multiple pathways leading to cell death. Both the primary aberrant pathway and compensatory pathways are inhibited.
Analyses using an independent, iterative screening and optimization process were used to assess combinations of targeted agents based on DTRM-12, a novel, covalent, selective BTK inhibitor that has been specifically designed as a backbone for combination therapies [2]. After in vitro and in vivo screening, the best candidate triplet was selected for clinical trials. Preclinical studies showed that concurrent BTK inhibition, mTOR inhibition and the use of an immunomodulating agent at low doses can synergistically kill malignant B cells. The DTRM-555 regimen was designed as an oral, once-daily, triplet combination consisting of DTRM-12, the mTOR inhibitor everolimus, and the immunomodulating agent pomalidomide.
A phase I clinical trial was conducted to test this concept in patients with B-cell non-Hodgkin lymphomas (NHLs) or CLL for whom no standard therapies were available. In stage 1 of the trial, DTRM-12 doses were escalated between 50 and 300 mg/d. Stage 2 was devoted to testing DTRM-12 in escalating doses plus everolimus 5 mg/d. Finally, in stage 3, the combination of DTRM-12 in escalating doses plus everolimus 5 mg/d and pomalidomide 2 mg/d on 21 out of 28 days was examined. The ultimate goal of the phase II was the production of a single, fixed-dose combination tablet.
Viable regimen in RT and DLBCL
At ASH 2020, Mato et al. presented the data on patients enrolled in phase I with a diagnosis of RT (n = 13) or de novo DLBCL (n = 11) [2]. All patients were pretreated with an anti-CD20 antibody, and all DLBCL patients had received R-CHOP, as had 69 % of RT patients. BTK inhibitors and the Bcl-2 inhibitor venetoclax had also been used in substantial proportions prior to study entry.
DTRM-12 200 mg/d, everolimus 5 mg/d and pomalidomide 2 mg/d were established as the recommended phase II dose of the DTRM-555 regimen. This combination had an acceptable safety profile, which meant that the primary endpoint of the study was met. The main safety findings were expected and manageable. Mostly, hematologic AEs occurred, with grade 3/4 neutropenia and grade 3/4 thrombocytopenia emerging in 54 % and 37 %, respectively. No patient discontinued combination therapy due to an AE. Pharmacokinetic analyses demonstrated that plasma concentrations of DTRM-12 were linear and unaffected by the coadministration of everolimus and pomalidomide. The pharmacokinetic data supported the once-daily dosing of DTRM-12, with an estimated half-life of 5–9 hours.
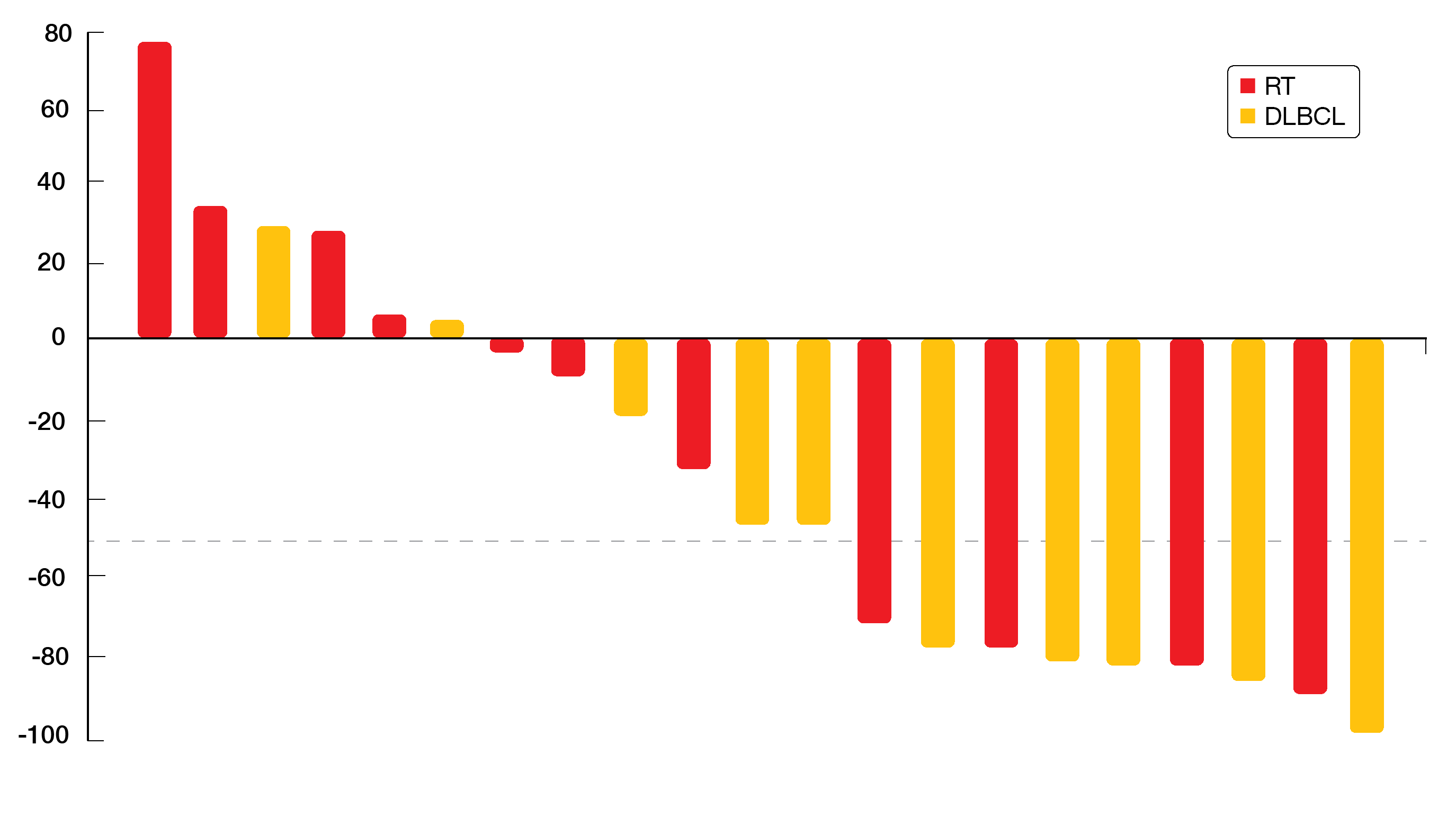
Best overall responses were 46 % and 45 % for patients with RT and DLBCL, respectively. In the RT population, 1 and 4 patients achieved CR and PR, respectively. For the DLBCL group, this applied to 2 and 3 individuals, respectively. Overall, 43 % of patients had ≥ 50 % reductions in the sum of the products of the greatest diameters of lymph nodes (Figure). Even patients with 10 prior treatment lines experienced durable responses. Overall, the triple fixed-dose combination tablet DTRM-555 showed encouraging clinical activity in a high-risk, heavily pretreated RT/DLBCL population. The tablet is currently in the final stage of development. Once-daily, oral dosing provides a convenient treatment schedule for patients and might therefore improve adherence to therapy.
Figure: Depth of nodal response observed with DTRM-555 treatment in patients who had Richter’s transformation or de novo DLBCL
Safety of zanubrutinib after ibrutinib/acalabrutinib
A multicenter, single-arm, open-label phase II study evaluated the next-generation BTK inhibitor zanubrutinib in 60 patients with previously treated B-cell malignancies who had discontinued ibrutinib and/or acalabrutinib due to AEs [3]. Tolerability issues are a common cause of treatment discontinuation with respect to these two agents [4, 5]. The primary objective was the assessment of the safety of zanubrutinib compared with the ibrutinib and/or acalabrutinib intolerance AE profile. Due to its improved selectivity, zanubrutinib was assumed to provide greater tolerability with reduced recurrence and severity of BTK-inhibitor–emergent AEs. The population included 25 patients with CLL/SLL, two with mantle cell lymphoma (MCL), and five with Waldenström’s macroglobulinemia (WM).
According to the analysis, zanubrutinib was tolerable and effective. Intolerable AEs experienced on ibrutinib and/or acalabrutinib were unlikely to recur while on zanubrutinib; no recurrence was noted for 88 % of ibrutinib-intolerant events and 50 % of acalabrutinib-intolerant events. Among the events that did recur, 88 % of ibrutinib events and 50 % of acalabrutinib events showed lower severity. None of the grade 4 intolerant events recurred on zanubrutinib. Out of 25 grade 3 events, only 2 recurred. No serious AEs were observed, and no patient discontinued treatment due to adverse events.
Compared with prior BTK inhibitor treatment, zanubrutinib maintained responses (44.4 %) or improved them (50 %). At a median follow-up of 3.5 months, 96.9 % of patients remained on study. As the authors noted, these data suggest that zanubrutinib might provide a therapeutic option in patients intolerant to other BTK inhibitors.
LOXO-305: durable responses in MCL
The highly potent and selective non-covalent BTK inhibitor LOXO-305 was assessed in 323 patients with CLL/SLL, MCL, WM, and other NHLs participating in the phase I/II BRUIN study. At ASH 2020, Wang et al. presented the efficacy results for 61 patients with MCL, 26 with WM and 66 with other NHLs, and safety results for all 323 patients [6].
In the efficacy population, all patients were pretreated. The median numbers of prior lines of systemic therapies were 3, 3, and 4 for those with MCL, WM and other NHLs. Previous treatments included all available classes of therapy including BTK inhibition, chemotherapy, anti-CD20 antibodies, CAR-T cell therapy, and stem cell transplantation. Progressive disease was the reason for discontinuation of BTK inhibitor pretreatment in 67–79 % of cases.
The pharmacokinetic analysis was conducted within a dose range from 25 to 300 mg/d. Here, plasma exposures of LOXO-305 were shown to be dose-dependent and linear. They exceeded BTK IC90 throughout dosing intervals at doses ≥ 100 mg/d. LOXO-305 showed a favorable safety profile consistent with its design as a highly selective and non-covalent BTK inhibitor. No dose-limiting toxicities were reported, and no maximum tolerated dose was identified. LOXO-305 200 mg/d was selected as the recommended phase II dose. Rates of AEs of special interest such as bruising, rash, arthralgia and hemorrhage were low. Among 323 patients, only 5 (1.5 %) discontinued LOXO-305 due to treatment-related AEs.
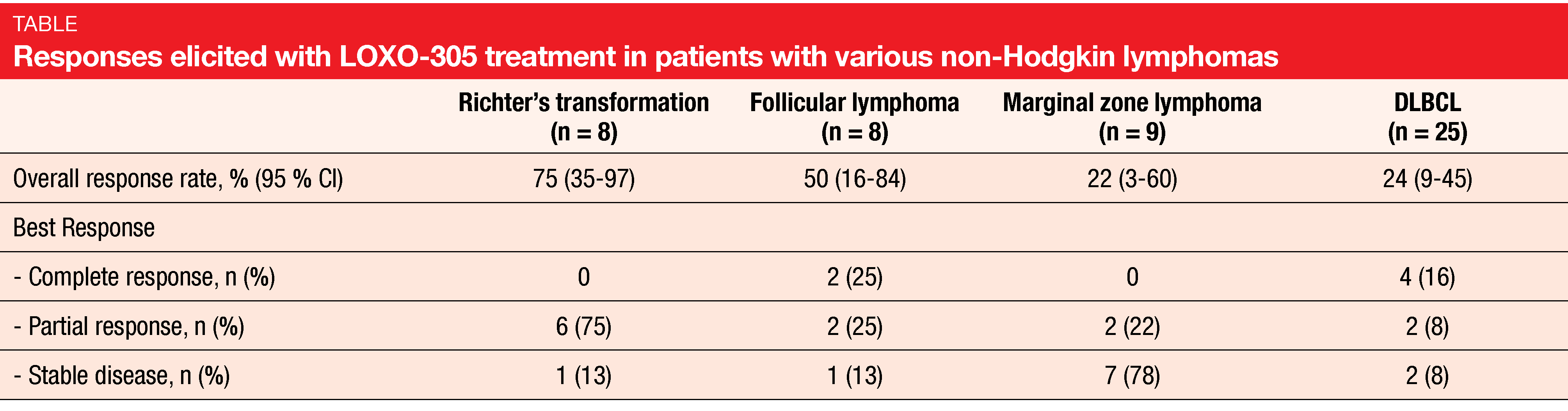
LOXO-305 demonstrated promising efficacy independent of prior therapy. ORRs were 52 % and 68 % for patients with MCL and WM, respectively. In the group with other NHLs, they ranged from 22 % for marginal zone lymphoma to 75 % for Richter’s transformation (Table). Notably, LOXO-305 led to durable responses in BTK-pretreated patients with MCL in whom outcomes are generally poor following progression on covalent BTK inhibitors such as ibrutinib [7, 8]. BTK-pretreated MCL patients achieved complete remissions in 25 %, and 83 % of responders were still responding and on treatment at 6 months. Overall, the analysis showed that LOXO-305 is well tolerated and exhibits promising efficacy in heavily pretreated patients with various NHLs. A longer follow-up is required to better understand the safety profile associated with chronic administration.
TG-1701 as single agent and in combination with U2
Another investigational next-generation BTK inhibitor is TG-1701, a once-daily, covalently bound agent. Compared with ibrutinib, it was shown to exhibit superior selectivity [9]. The triple combination of TG-1701 with the dual PI3Kδ/CK-1ε inhibitor umbralisib and ublituximab, a glycoengineered anti-CD20 antibody, demonstrated inhibition of tumor growth in BTK-resistant xenograft models [10]. Cheah et al. reported phase I study results for TG-1701 as monotherapy and in combination with umbralisib and ublituximab (U2) in patients with B-cell lymphoma or CLL that warranted systemic therapy [11]. Parallel dose escalation was conducted in both monotherapy (n = 25; 100–400 mg/d) and combination cohorts (n = 16; 100–300 mg/d). Disease-specific cohorts were expanded at 200 mg and 300 mg of single-agent TG-1701 for patients with CLL (n = 20), MCL (n = 21), and WM (n = 20). All patients in the dose escalation phase had relapsed and refractory disease, while several individuals in each disease-specific cohort were treatment-naïve.
The safety profile was generally favorable, and no patient discontinued therapy due to AEs. For both TG-1701 monotherapy and the triplet combination, grade ≥ 3 AEs were infrequently observed. Regarding efficacy, monotherapy at doses of 100–400 mg gave rise to an ORR of 52 %. In the disease-specific cohorts treated with TG-1701 200 mg, ORRs for patients with CLL, MCL and WM were 95 %, 50 %, and 95 %, respectively. For the dose-escalation triplet combination consisting of TG-1701 and U2, the ORR was 79 %, including complete remissions in 22 %. At the time of the analysis, most patients were still on study.
Overall, TG-1701 exhibited an encouraging safety profile, with clinical and pharmacodynamic activity at all evaluated dose levels that support once-daily dosing. The maximum tolerated dose had not been reached in the monotherapy arm. Also, the combination with U2 was well tolerated, and dose escalation continues. Combination treatment was associated with encouraging clinical activity, including early complete responses. This study continues enrollment, and future registrations trials are being planned.
REFERENCES
- Tsimberidou AM, Keating MJ, Richter syndrome: biology, incidence, and therapeutic strategies. Cancer 2005; 103(2): 216-228
- Mato AR et al., A once daily, oral, triple combination of BTK inhibitor, mTOR inhibitor and IMID for treatment of relapsed/refractory Richter’s transformation and de novo diffuse large B-cell lymphoma. ASH 2020, abstract 126
- Shadman M et al., Phase 2 study of zanubrutinib in patients with previously treated B-cell malignancies intolerance to ibrutinib/acalabrutinib. ASH 2020, abstract 2947
- Mato AR et al., Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica 2018; 103(5): 874-879
- Yazdy MS et al., Toxicities and outcomes of acalabrutinib-treated patients with chronic lymphocytic leukemia: a retrospective analysis of real world patients. Blood 2019; 134(Supplement_1): 4311
- Wang ML et al., LOXO-305, a next generation, highly selective, non-covalent BTK inhibitor in previously treated mantle cell lymphoma, Waldenström’s macroglobulinemia, and other Non-Hodgkin lymphomas: results from the phase 1/2 BRUIN study. ASH 2020, abstract 117
- Cheah CY et al., Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol 2015; 26: 1175-1179
- Martin P et al., Post ibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016; 127: 1559-1563
- Normant E et al., TG-1701, a novel, orally available, and covalently bound BTK inhibitor. EHA 2018, abstract PF638
- Ribeiro ML et al., TG-1701, a novel irreversible Bruton’s kinase inhibitor, cooperates with ublituximab-driven ADCC and ADCP in in vitro and in vivo models of ibrutinib-resistant mantle cell lymphoma. AACR 2020, abstract 2205
- Cheah CY et al., Clinical activity of TG-1701, as monotherapy and in combination with ublituximab and umbralisib, in patients with B-cell malignancies. ASH 2020, abstract 1130
© 2020 Springer-Verlag GmbH, Impressum