Management of CLL patients: BTK inhibition and beyond
BTK inhibitors, the Bcl-2 inhibitor venetoclax and anti-CD20 antibodies such as obinutuzumab have dramatically changed the therapeutic landscape of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Ibrutinib, as the first-generation representative of the BTK inhibitor class, is a therapeutic mainstay, although it has notable shortcomings that led to the introduction of second-generation agents. Acalabrutinib has already received approval in the US and Europe for the treatment of patients with CLL, while zanubrutinib was approved in China in this indication in 2020. BTK-inhibitor–based doublets and triplets are being evaluated in clinical trials, particularly with a view to achieving undetectable minimal residual disease that allows for fixed-duration therapy and treatment discontinuation. At the same time, new agents such as orelabrutinib and LOXO-305 are being tested in the clinical setting. Progress is also ongoing with respect to the expansion of PI3Kδ inhibitory options and the development of novel anti-CD20 antibodies.
Ibrutinib-based therapy
CAPTIVATE: ibrutinib plus venetoclax
To date, ibrutinib is the only targeted therapy to demonstrate significant OS benefit as a first-line treatment of patients with CLL included in randomized phase III studies [1, 2]. The international, phase II CAPTIVATE trial evaluated 12 cycles of ibrutinib plus venetoclax in the first-line setting to explore the question of whether deep remission can be achieved with 1-year fixed treatment duration to allow for treatment discontinuation. At ASH 2020, Wierda et al. presented the primary analysis for the minimal residual disease (MRD) cohort [3]. In this cohort (n = 149), treatment with ibrutinib plus venetoclax for 12 cycles after a 3-month lead-in with ibrutinib only was followed by randomization based on the MRD status. Patients with confirmed undetectable MRD (uMRD) received either placebo or ibrutinib in a double-blind manner, while those in whom uMRD was not confirmed underwent open-label randomization to either ibrutinib or ibrutinib plus venetoclax. The primary endpoint was the 1-year disease-free survival (DFS) rate in patients with confirmed uMRD randomized to placebo vs. ibrutinib.
Fifty-eight percent of patients in the MRD cohort achieved confirmed uMRD in the peripheral blood and bone marrow after 12 cycles of ibrutinib and venetoclax. Within this group, the 1-year DFS rates did not differ significantly between placebo and ibrutinib after a median follow-up of 16.6 months post-randomization (100 % vs. 95.3 % for ibrutinib and placebo, respectively; p = 0.1475). According to the authors, the 95 % rate in the placebo arm supports a fixed-duration treatment approach and discontinuation in patients who obtained confirmed uMRD. The overall median follow-up on the study was 31.3 months. At 30 months, the PFS rates were > 95 % across all four randomized arms. This compared favorably to other first-line fixed-duration regimens including rituximab, fludarabine and cyclophosphamide (3-year PFS, 73 %) [2] and venetoclax plus obinutuzumab (3-year PFS, 82 %) [4].
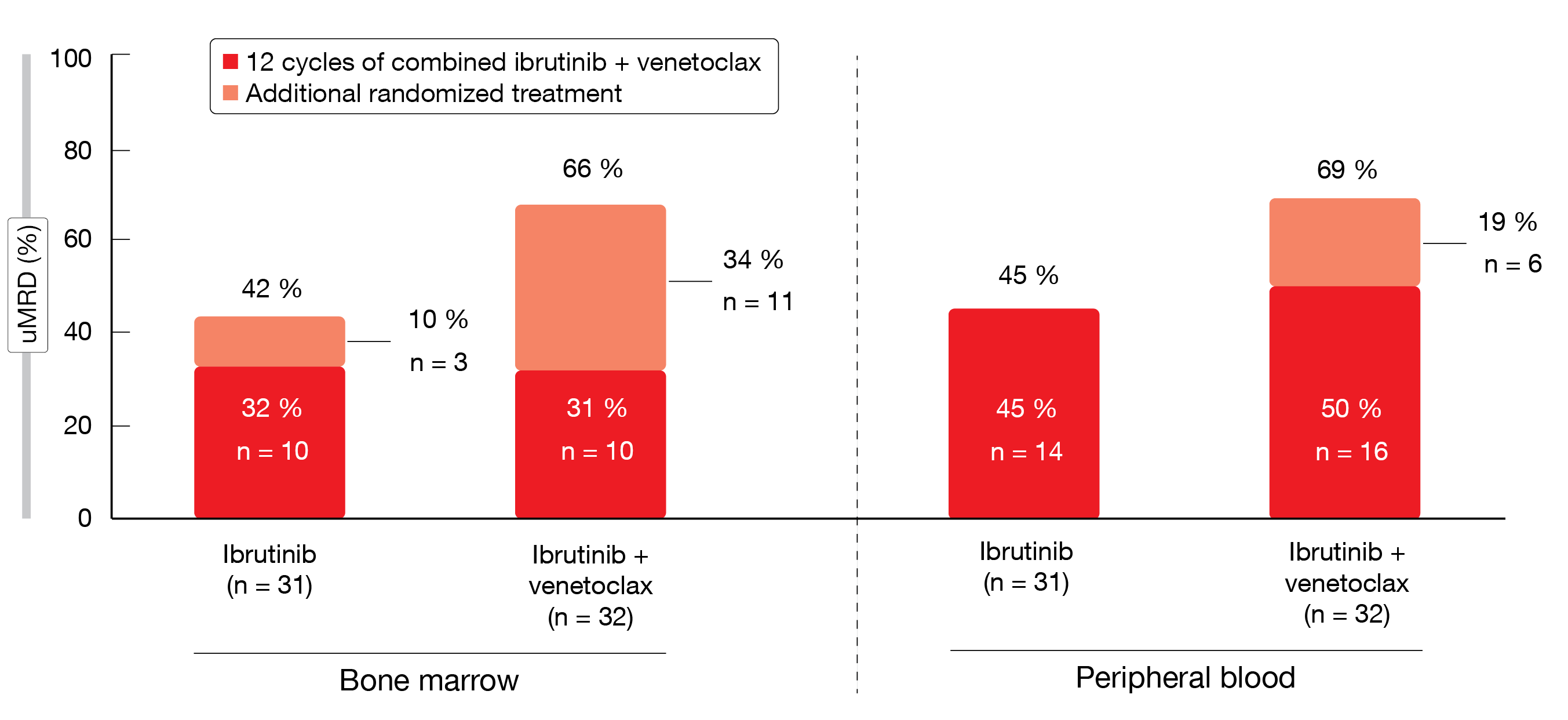
For patients who did not achieve confirmed uMRD at the end of 12 cycles of ibrutinib plus venetoclax, improvement in MRD status was observed with continued administration of the combination vs. single-agent ibrutinib. In the bone marrow, the additional randomized treatment led to a 34 % increase in uMRD in patients receiving ibrutinib plus venetoclax, whereas this was only 10 % in the ibrutinib-only arm (Figure 1). The uMRD rate in the peripheral blood increased by 19 % in the combination arm vs. 0 % in the monotherapy arm. AEs generally lessened after the first 6 months of ibrutinib plus venetoclax treatment irrespective of the subsequent randomized regimen. Few patients required dose adjustment or discontinuation. Grade ≥ 3 AEs were uncommon. Based on these findings, the authors concluded that ibrutinib plus venetoclax is an all-oral, once-daily, chemotherapy-free, fixed-duration regimen that provided highly concordant, deep MRD remissions in bone marrow and blood in first-line CLL.
Figure 1: Increases in best overall uMRD rates with additional randomized treatment in the uMRD not confirmed population of the CAPTIVATE study
MRD-guided treatment intensification
A single-arm, phase II study including patients with relapsed/refractory (r/r) CLL who were naïve to BTK and Bcl-2 inhibitors investigated the efficacy of the addition of ibrutinib to venetoclax in terms of MRD [5]. Thirty-eight patients received venetoclax for 12 months; at that time, response and MRD status were evaluated. Those who had achieved complete response (CR)/partial response (PR) and uMRD stopped treatment, while those with CR/PR and detectable MRD continued venetoclax therapy and added ibrutinib. MRD was periodically evaluated, and patients who obtained uMRD in both peripheral blood and bone marrow at any time discontinued treatment. Patients with detectable MRD at the end of the 12-month combination period went on to receive single-agent ibrutinib.
This sequential MRD-guided approach proved to be feasible. uMRD was obtained by 45 % of patients after 12 months of venetoclax monotherapy. Among the remaining patients, 84 % achieved uMRD with venetoclax plus ibrutinib. In total, MRD was achieved in 87 % with either the monotherapy or the combination. Overall, 95 % of patients responded. Only 2 clinical relapses occurred after uMRD during the 27-month follow-up. The authors noted that responses to venetoclax retreatment remain to be established; also, the biological characteristics of patients with persistent MRD and early MRD relapse after treatment discontinuation will be investigated.
Obinutuzumab, ibrutinib & venetoclax
The fixed-duration triple combination of ibrutinib, venetoclax and obinutuzumab was tested in a phase II study enrolling patients with treatment-naïve (n = 25) and r/r CLL (n = 25). Obinutuzumab was administered in cycles 1-8, ibrutinib in cycles 2-14, and venetoclax in cycles 3-14. Rogers et al. presented the 3-year update at the ASH 2020 Congress [6]. The CR rates with uMRD (i.e., the primary endpoint) were 28 % in both treatment-naïve and r/r patients. Fifty-six percent and 44 %, respectively, showed uMRD in both blood and bone marrow. Overall, 84 % and 88 %, respectively, achieved PR, CR or CR with incomplete marrow recovery (CRi). At 36 months, the PFS rate was 95 % in both groups, and OS rates amounted to 95 % and 100 % for treatment-naïve and r/r patients, respectively. These findings indicated durability of responses with the triplet therapy. Two cases of disease progression occurred in the r/r group, at 24 and 36 months.
This suggested that a time-limited treatment of 14 cycles with the three-drug combination can result in continued durable remissions. Additional follow-up is required to determine the median PFS and duration of benefit to patients. Ibrutinib plus venetoclax and obinutuzumab is currently being compared to ibrutinib plus obinutuzumab in two phase III cooperative group trials (NCT03701282 and NCT03737981).
Regimens including second-generation BTK inhibitors
Acalabrutinib: final results of ASCEND
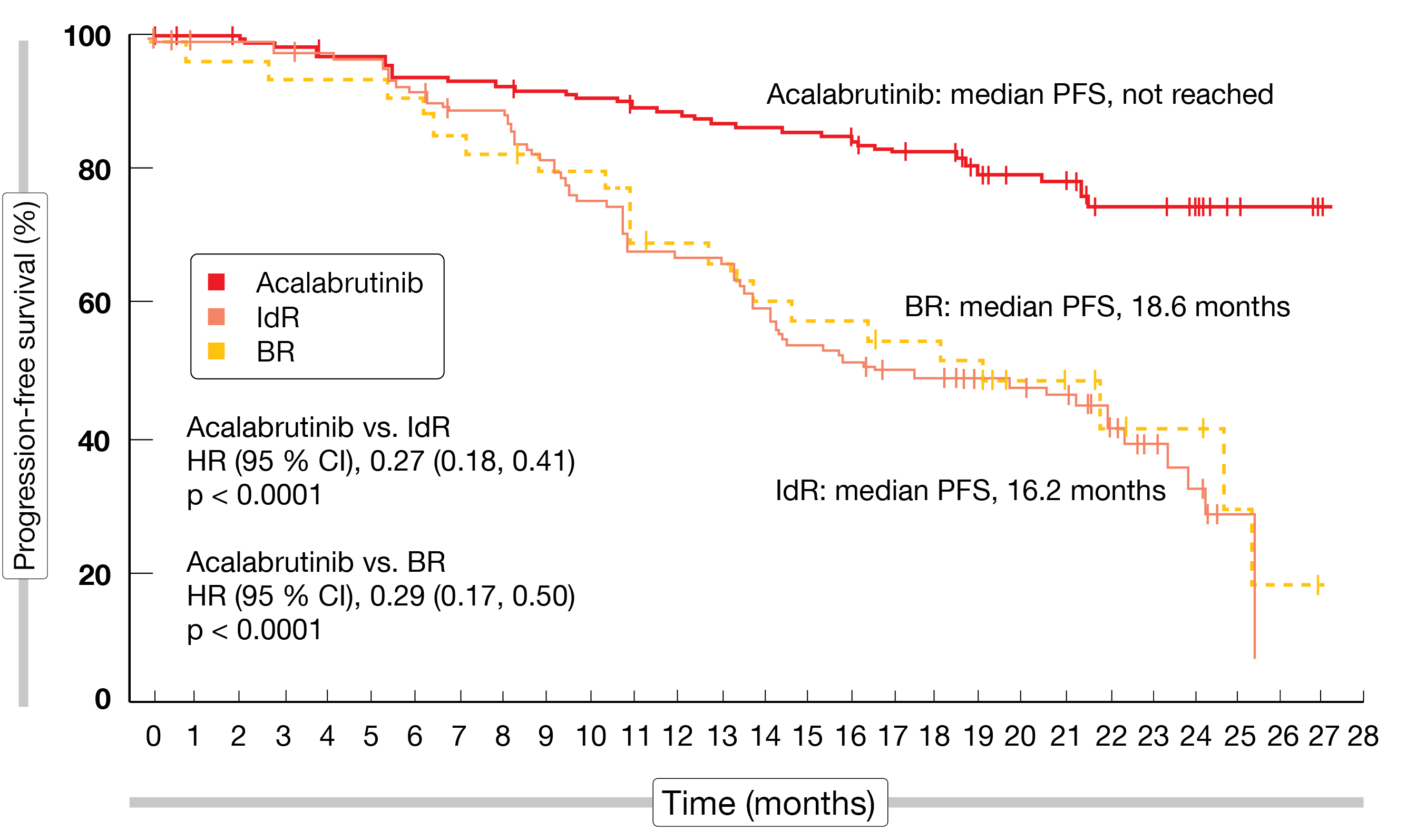
At ASH 2020, Ghia et al. reported the final results of the ASCEND trial that compared the second-generation, highly selective, covalent BTK inhibitor acalabrutinib (n = 155) with idelalisib plus rituximab (IdR; n = 119) or bendamustine plus rituximab (BR; n = 36) according to investigator’s choice in patients with r/r CLL [7]. After a median follow-up of 22 months, the results emphasized the favorable efficacy and safety of the BTK inhibitor compared to standard-of-care regimens. Median PFS had not been reached with acalabrutinib, while this was 16.2 months with IdR and 18.6 months with BR (p < 0.0001 for both comparisons; Figure 2). At 18 months, PFS rates were 82 % vs. 48 % for acalabrutinib and IdR/BR, respectively. PFS benefits were also observed in patients with high-risk genetic features including deletion 17p, TP53 mutations and unmutated IGHV status. Median OS had not been reached yet in either treatment arm. The overall response rates (ORRs) did not differ (80 % vs. 84 %), although the median duration of response was longer with acalabrutinib than with IdR/BR (not reached vs. 18 months; HR, 0.19). At 18 months, 85.4 % vs. 49.4 % of patients responded.
Both acalabrutinib and BR showed higher tolerability than IdR. Compared to IdR, grade ≥ 3 AEs, treatment-related AEs and AEs leading to drug discontinuation or dose delays occurred less frequently with acalabrutinib and BR. As the authors summarized, these data support the use of acalabrutinib in patients with r/r CLL including those with high-risk features.
Figure 2: Superior progression-free survival for acalabrutinib versus bendamustin/rituximab (BR) and idelalisib/rituximab (IdR)
Triple acalabrutinib combinations: phase Ib
Acalabrutinib was tested in various combinations in the phase Ib ACE-CL-003 study. Woyach et al. presented data on two cohorts of the trial, namely cohort 3 evaluating acalabrutinib plus venetoclax and rituximab (AVR) in patients with r/r CLL and cohort 4 that assessed acalabrutinib plus venetoclax and obinutuzumab (AVO) in the treatment-naïve setting [8]. Each cohort contained 12 patients. With regard to safety, which was the primary endpoint, AEs turned out as expected based on each individual agent’s safety profiles. One patient in either cohort discontinued treatment due to AEs. Headache, diarrhea and nausea were the most frequent AEs of all grades. Most AEs of interest including infections, hemorrhage, neutropenia, and hypertension were low-grade. No tumor lysis syndrome (TLS) occurred.
After 16 cycles, the ORRs were 92 % and 100 % for AVR and AVO, respectively. Half of patients in each cohort had achieved CR or CRi at the time of data cutoff. All of those with CR/CRi obtained uMRD in the blood at the time of CR/CRi or earlier. At cycle 10, only 1 of 12 patients (8 %) in each cohort remained MRD-positive in the blood. Six cycles later, none of the patients in the AVR cohort and one patient in the AVO cohort were MRD-positive. The overall uMRD rate was 71 % (67 % and 75 % in patients treated with AVR and AVO, respectively). Median duration of response, PFS, and OS had not been reached in either group.
The authors concluded that the triple combination therapy with acalabrutinib plus an anti-CD20 antibody and a Bcl-2 inhibitor is feasible based on tolerability and yielded high CR and uMRD rates in both r/r and treatment-naïve patients with CLL.
Phase II data on AVO in treatment-naïve patients
AVO as front-line CLL treatment is currently being assessed in a phase II trial. Acalabrutinib is administered for 15 cycles, obinutuzumab from cycle 2 to 8, and venetoclax from cycle 4 to 15. If CR with uMRD has been obtained after the 15th cycle, acalabrutinib and venetoclax are discontinued but can be resumed upon MRD positivity. The MRD status is tested every 3 months. Patients who continue to show MRD-positive CR and/or PR after 15 cycles receive the doublet regimen for another 8 cycles. This is followed by a response reassessment and the same allocation, with acalabrutinib and venetoclax administered until progression in case of MRD positivity. Stable disease or progression prompt patient removal from the study. The study population is enriched for high-risk disease, with substantial proportions of patients showing unmutated IGHV status, TP53 mutation, and other aberrations. A recent protocol amendment restricted additional enrollment to patients with TP53-aberrant disease in a new cohort.
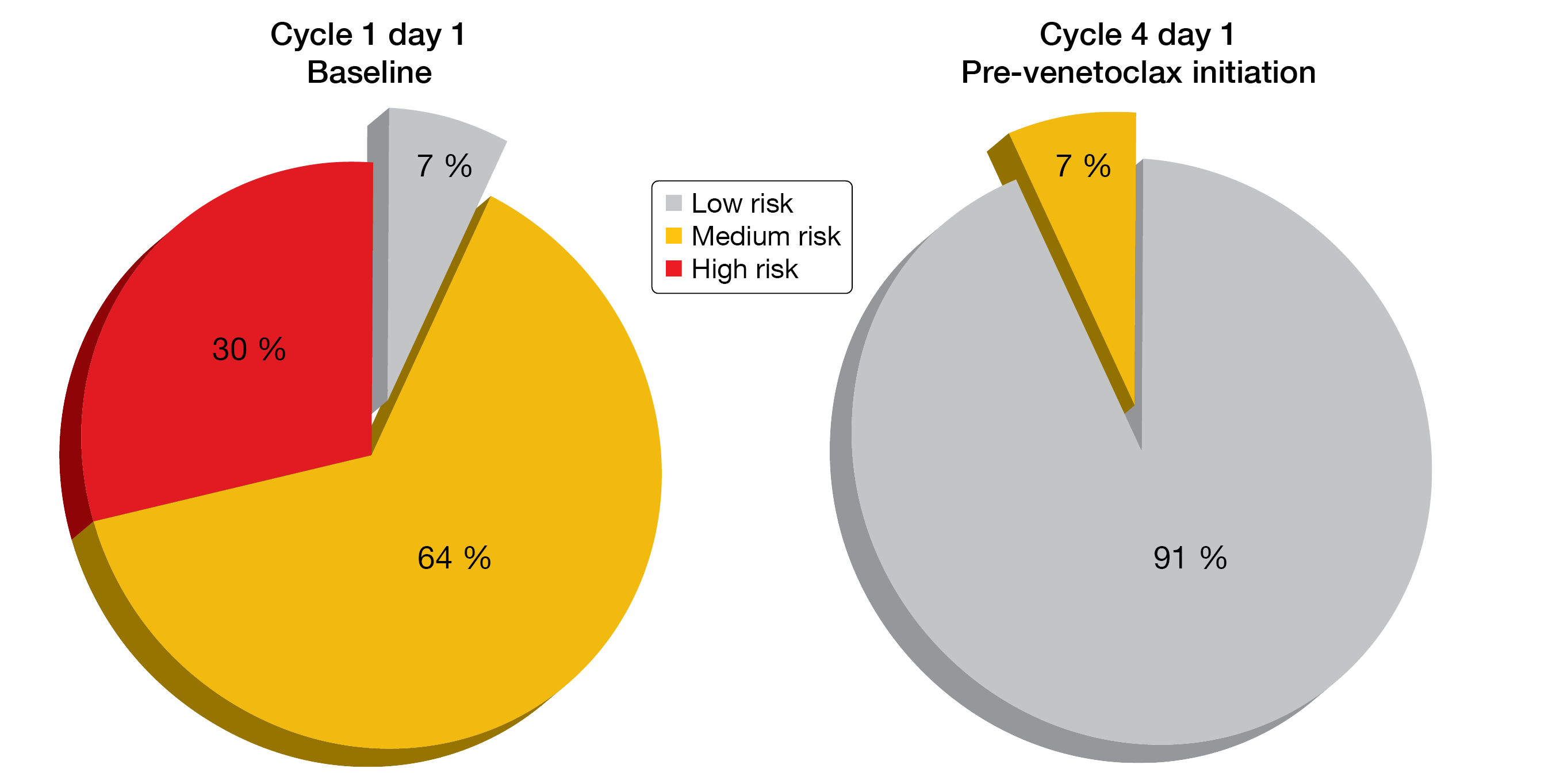
The updated analysis reported at ASH 2020 yielded uMRD rates of 76.5 % and 83.9 % in bone marrow and blood, respectively, after 15 cycles of therapy in the overall cohort (n = 44) [9]. In patients with TP53 aberrations (n = 17), these were 70 % and 90 %, respectively. After a median follow-up of 19 cycles, no patients had progressed or developed recurrent MRD. The depth of response increased over time. At cycle 16, CR/CRi rates were 44 % and 40 % in the total cohort and in the patients with TP53 aberrations, respectively. AVO showed a favorable safety profile with low risk of grade ≥ 3 infections, atrial fibrillation, and infusion-related reactions (2 % each). The 3-cycle lead-in with acalabrutinib and obinutuzumab effectively reduced TLS risk at the time of venetoclax initiation (Figure 3). No TLS event was observed due to venetoclax. A phase III trial of AVO vs. AV vs. chemoimmunotherapy is currently recruiting (NCT03836261).
Figure 3: Reduction in tumor lysis syndrome risk based on the 3-cycle lead-in with acalabrutinib and obinutuzumab
Meta-analysis of time-limited vs. continuous agents
Molica et al. performed a systematic literature review and network meta-analysis to estimate the relative efficacy and safety of targeted agents approved by the FDA and/or EMA for upfront therapy of CLL (i.e., ibrutinib, acalabrutinib, and venetoclax) [10]. In particular, time-limited venetoclax-based regimens were compared with continuous BTK inhibitor-based therapy based on the following trials: ILLUMINATE (ibrutinib + obinutuzumab; IO), CLL14 (venetoclax + obinutuzumab; VO), and ELEVATE-TN (acalabrutinib monotherapy and acalabrutinib + obinutuzumab; AO). Data were available for a total population of 1,191 individuals.
PFS did not differ between time-limited (VO) and continuous therapy (IO and acalabrutinib monotherapy). However, those treated with AO fared better with respect to PFS than the groups receiving IO and VO. A subgroup analysis focusing on patients with TP53 aberrations demonstrated similar PFS outcomes irrespective of the targeted agent used. Also, the incidence of grade 3–4 AEs did not vary significantly across ibrutinib, acalabrutinib and venetoclax treatment. Ongoing studies will further delineate the position of different targeted therapies and schedule of administration in CLL therapy based on efficacy, availability, safety, cost, treatment objectives, and patient choice.
SEQUOIA: zanubrutinib in deletion 17p
Patients with CLL/SLL who harbor deletion 17p have an unfavorable prognosis and respond poorly to standard chemoimmunotherapy [11, 12]. BTK and Bcl-2 inhibitors have been shown to improve outcomes for patients with deletion 17p [13, 14]. However, the first-generation BTK inhibitor ibrutinib demonstrates limited efficacy in this population. Real-world data presented at ASH 2020 strengthen the evidence indicating inferior survival with first-line ibrutinib in deletion 17p-positive patients compared to those without deletion 17p, which reflects an ongoing need for more efficacious therapies [15].
The global, phase III, open-label, randomized SEQUOIA study aimed at evaluating first-line use of the second-generation BTK inhibitor zanubrutinib in CLL/SLL patients with and without deletion 17p. Zanubrutinib was designed to maximize BTK occupancy and minimize off-target inhibition of TEC and EGFR family kinases [16, 17]. Patients included in Arm C of the SEQUOIA trial had deletion 17p and received zanubrutinib monotherapy. Brown et al. presented the updated results for this group after a median follow-up of 22 months [18]. Among 109 enrolled patients, 95 were still on study treatment at the time of the analysis. Single-agent zanubrutinib gave rise to an ORR of 94.5 % and a CR/CRi rate of 6.4 % that had increased from the initial CR/CRi rate of 1.9 % estimated 1 year earlier [19]. Almost 88 % of patients showed ongoing responses for at least 18 months. The 18-month PFS and OS rates were 90.6 % and 95.4 %, respectively. PFS was analyzed by IGHV mutation and karyotype status. With limited follow-up, the findings appeared similar between patients with unmutated vs. mutated IGHV as well as between patients with complex vs. non-complex karyotype. The tolerability of zanubrutinib monotherapy was generally consistent with previous observations reported for patients with various B-cell malignancies [17, 20-22].
Based on the encouraging results obtained in Arm C, Arm D of the SEQUOIA study was devised to evaluate zanubrutinib plus venetoclax [23]. At present, patients are being recruited into this arm in 8 countries worldwide, with planned enrollment of approximately 50 individuals. After 3 months of zanubrutinib monotherapy, venetoclax is added for 12-24 cycles. Restaging and MRD measurements are performed regularly at 3-monthly intervals. Patients who achieve confirmed uMRD in blood and bone marrow are allowed to discontinue zanubrutinib and venetoclax therapy after a minimum of 27 and 12 cycles, respectively.
Zanubrutinib, obinutuzumab, and venetoclax: BOVen
Ibrutinib plus venetoclax doublets as well as triplets with obinutuzumab are active but associated with characteristic toxicities. The BOVen study was conducted based on the hypothesis that first-line treatment with zanubrutinib, obinutuzumab, and venetoclax (BOVen) will achieve frequent uMRD, and MRD-driven treatment discontinuation will allow for durable responses off treatment [24]. Venetoclax was introduced in cycle 3 after a 2-month lead-in with zanubrutinib and obinutuzumab. Patients completed 8 cycles of obinutuzumab and 6 cycles of the triple combination. Thereafter, the management was determined by pre-specified MRD endpoints with a minimum and maximum duration of therapy of 8 and 24 months, respectively. If uMRD according to flow cytometry (< 10-4) was present, the patients received 2 additional cycles. In case of ongoing confirmed uMRD at the time of the next blood MRD test, they discontinued therapy with the option for retreatment at progression. The frequency of uMRD confirmed in blood and marrow constituted the primary outcome.
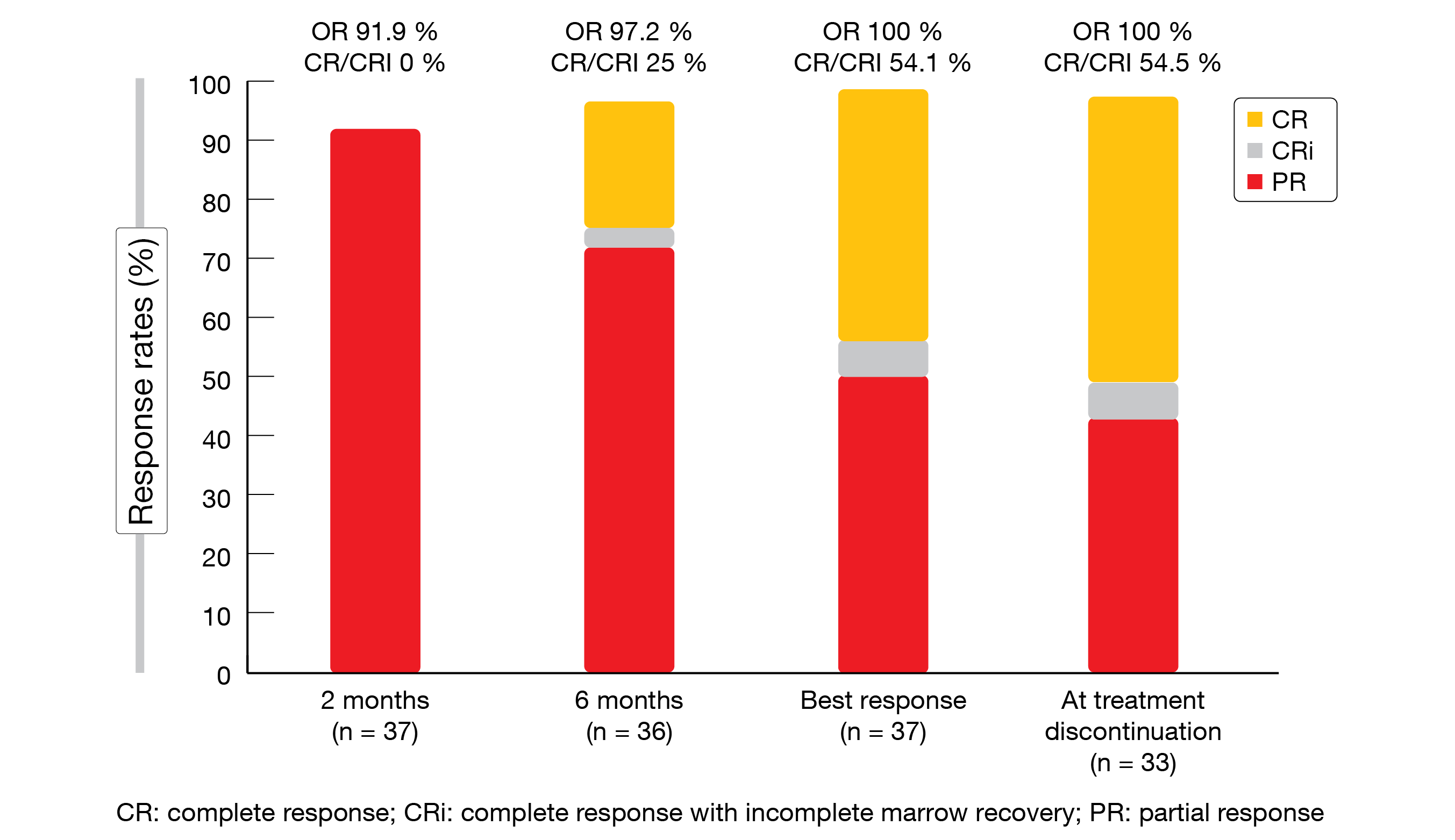
In 89.2 % of 37 evaluable patients, uMRD was achieved in both blood and marrow, and the treatment was discontinued after a median of 10 months. All of the patients responded, with 54.5 % achieving CR/CRi at the time of treatment discontinuation (Figure 4). BOVen was well tolerated; only few grade ≥ 3 treatment-emergent AEs occurred. Among 34 patients who achieved uMRD in peripheral blood by flow cytometry, 97 % obtained uMRD according to immunosequencing at a cutoff of < 10-5. Rapid clearance with a ≥ 400-fold decrease within 6 cycles of starting the BOVen triplet was highly predictive of uMRD and might thus identify a favorable patient group.
Figure 4: Response rates according to iwCLL criteria observed in the BOVen study
Early vs. late use of zanubrutinib
Pooled data from two phase I studies (NCT02343120 and NCT03189524) and one phase II study (NCT03206918) investigating zanubrutinib monotherapy in CLL/SLL patients showed that superior outcomes can be obtained with this agent in earlier lines [25]. Treatment-naïve patients had a significantly higher ORR than those with relapsed/refractory disease (100 % vs. 90.6 %; p < 0.001), and median PFS was numerically longer (HR, 0.32; p = 0.14). For OS, the comparison revealed no difference. In general, treatment-naïve patients showed a better exposure-adjusted safety profile that those with r/r CLL, particularly regarding AEs of special interest such as diarrhea, hypertension and atrial fibrillation/flutter.
Another comparison across patients with 1 prior line of treatment and those after ≥ 2 prior lines yielded similar results. Here, the ORR was numerically higher in the early lines (97 % vs. 88.3 %; p = 0.05), while median PFS was significantly longer (HR, 0.13; p < 0.001). The 24-month PFS rates amounted to 95 % and 75.3 %, respectively. Again, OS did not differ. Exposure-adjusted safety profiles were generally similar for both groups, although patients after only 1 treatment line showed lower rates of AEs of special interest.
Emerging BTK-inhibiting agents
Robust results for orelabrutinib
The ongoing, multicenter, open-label, single-arm, phase II ICP-CL-00103 study is evaluating the novel, highly selective, irreversible BTK inhibitor orelabrutinib in 80 patients with r/r CLL. According to the update provided by Xu et al. at the ASH 2020 Congress, the results confirmed the efficacy of orelabrutinib [26]. At a median follow-up of 14.3 months, the ORR according to independent review was 91.3 %, and CR/CRi was obtained in 10 %. Disease control resulted in 95 %. Over time, the CR rates increased significantly. Also, very high ORRs occurred in cytogenetic high-risk subgroups (deletion 17p, 100 %; TP53 mutation, 100 %; deletion 11q, 94.7 %; unmutated IGHV, 93.9 %).
Neither median PFS nor median duration of response had been reached at the time of data cutoff. At 12 months, the PFS rate was 81.1 %, and 77.1 % of patients responded. Orelabrutinib showed a robust safety profile and was found to be well tolerated with low rates of off-target side effects. Neutropenia, thrombocytopenia and upper respiratory tract infection occurred most commonly. No events were reported regarding atrial fibrillation/flutter and grade ≥ 3 hypertension. Grade ≥ 3 diarrhea occurred in one case, and major hemorrhage was seen in 2 cases.
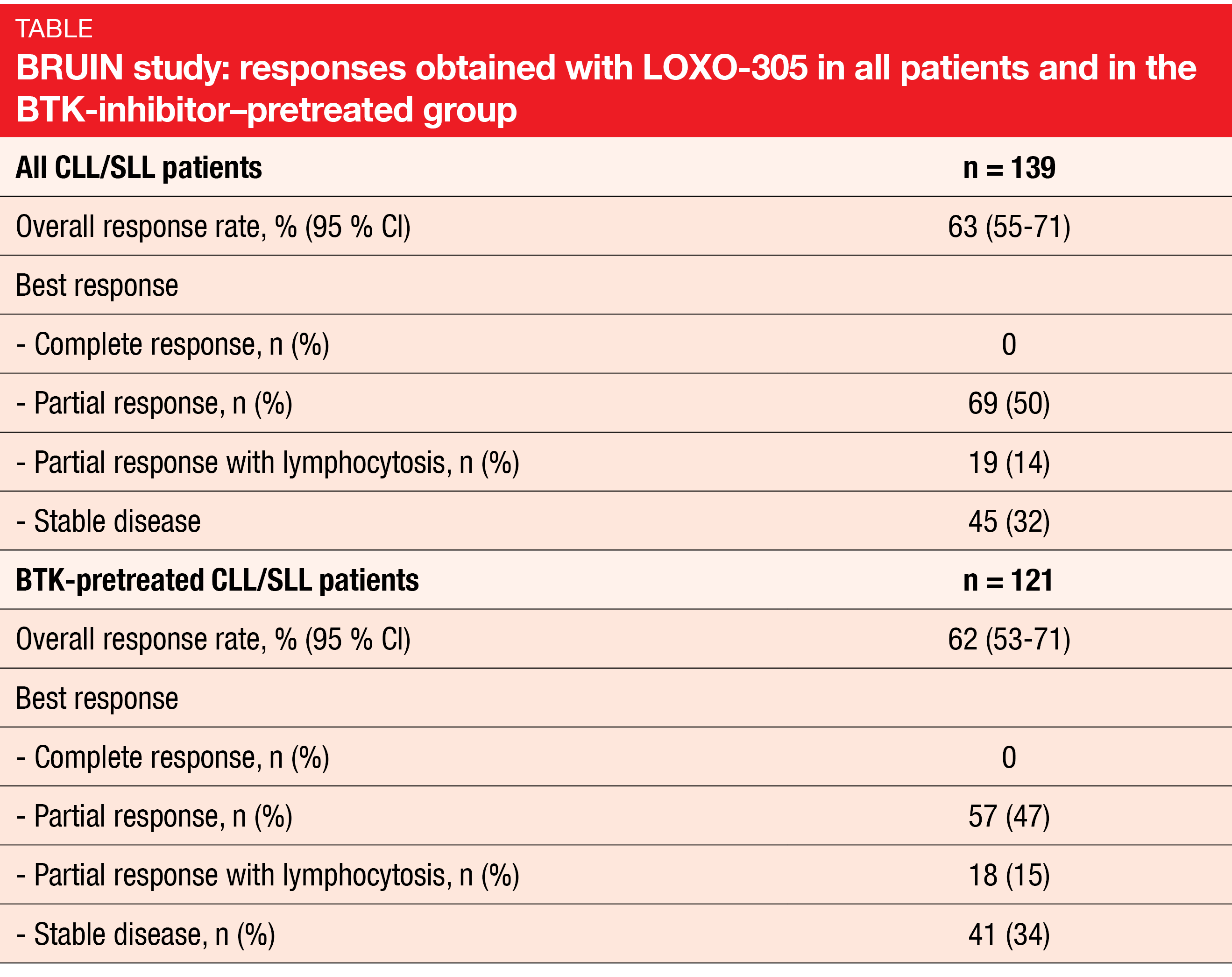
LOXO-305: BRUIN
The phase I/II BRUIN study demonstrated promising efficacy of the novel, non-covalent BTK inhibitor LOXO-305 in CLL/SLL patients previously treated with all classes of available therapy [27]. BRUIN included 170 patients whose number of prior lines of systemic therapy ranged from 1 to 11. Eighty-six percent had already received BTK inhibition, with progressive disease representing the most common reason for treatment discontinuation (67 %). High-risk molecular features were present in considerable percentages of patients.
The safety and tolerability of LOXO-305 were favorable and consistent with the design of this agent as a highly selective and non-covalent BTK inhibitor. No dose-limited toxicities occurred, and only 1.5 % of patients discontinued LOXO-305 due to treatment-related AEs. A daily dose of 200 mg was selected as the recommended phase II dose.
LOXO-305 showed efficacy regardless of BTK pretreatment and use of other prior agents, the reason for BTK inhibition discontinuation (i.e., progression vs. intolerance), and the presence of the BTK C481 mutation status. In the total population and the BTK-pretreated group, the ORRs were 63 % and 62 %, respectively (Table). Responses increased over time and were ongoing in 94 % of responders after a median follow-up of 6 months. Median PFS had not been reached yet.
Novel agents targeting PI3Kδ and CD20
UNITY-CLL
As not all patients are ideal candidates for BTK and Bcl-2 inhibitors, other agents such as phosphatidylinositol-3-kinase-delta (PI3Kδ) inhibitors with distinct mechanisms of action have been evaluated. However, studies of previous generations of PI3Kδ inhibitors in treatment-naïve CLL patients have shown substantial toxicity [28, 29]. Umbralisib, an oral dual inhibitor of PI3Kδ and casein kinase-1ε, exhibits improved selectivity for the delta isoform of PI3K, with low rates of immune-mediated toxicities and discontinuations due to AEs [30, 31]. The combination of umbralisib and ublituximab, which is a novel anti-CD20 monoclonal antibody offering enhanced antibody-dependent cellular cytotoxicity, has demonstrated promising activity in heavily pretreated CLL patients [32].
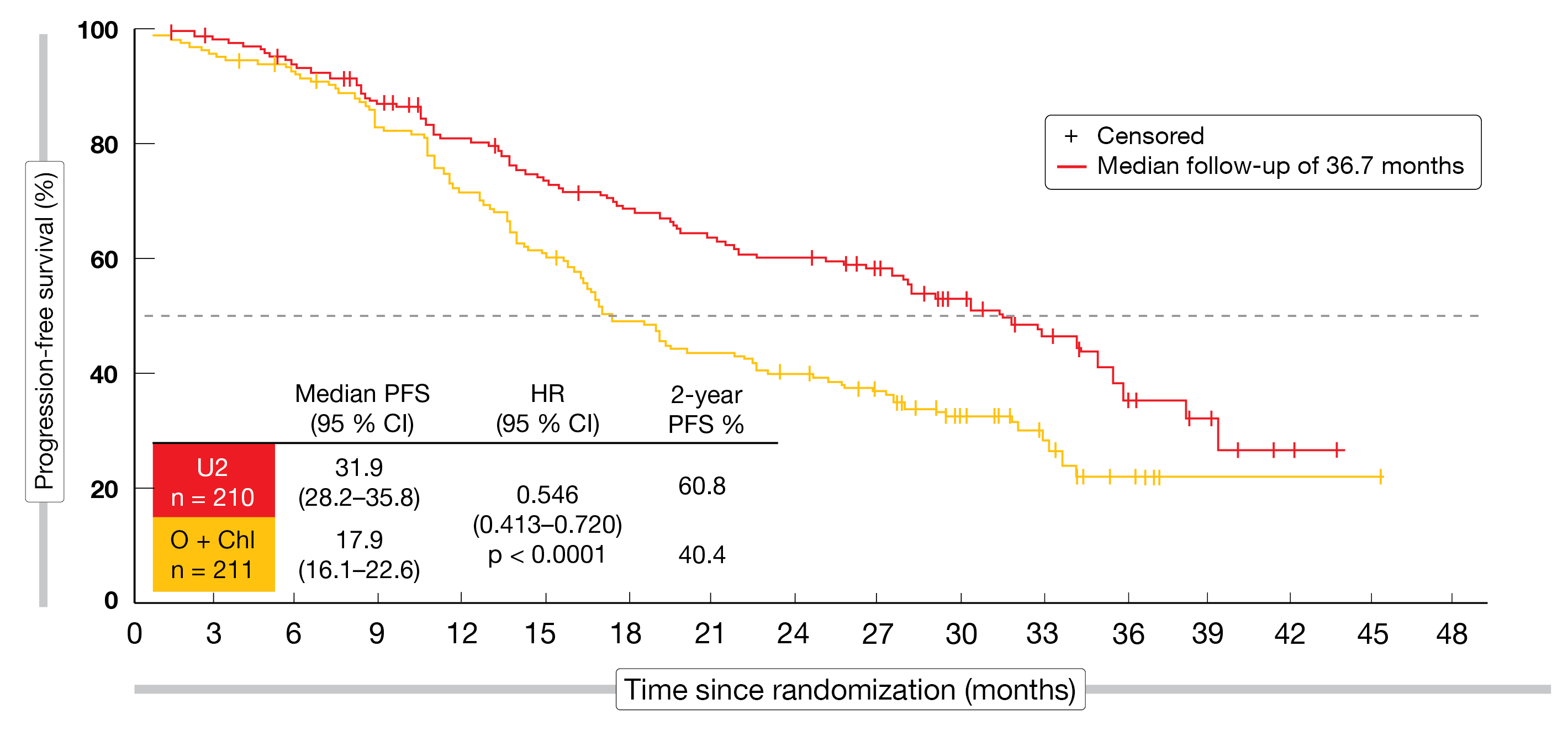
The UNITY-CLL study compared umbralisib plus ublituximab (U2; n = 210) with chemoimmunotherapy consisting of obinutuzumab and chlorambucil (n = 211) in patients with treatment-naïve or r/r CLL [33]. PFS according to independent review committee was defined as the primary endpoint. In both arms, 57 % and 43 % of patients were treatment-naïve and pretreated, respectively. The median numbers of prior therapies were 2 and 1 for U2 and obinutuzumab plus chlorambucil, respectively.
Durable responses regardless of subgroup
In terms of the primary endpoint, the novel combination was superior to obinutuzumab plus chlorambucil in the ITT population, with a median PFS of 31.9 vs. 17.9 months (HR, 0.546; p < 0.0001; Figure 5). At 2 years, the PFS rates were 60.8 % vs. 40.4 %. The treatment-naïve population derived greater PFS benefits in both arms (38.5 vs. 26.1 months; HR, 0.482; p < 0.001) than the pretreated population (19.5 vs. 12.9 months; HR, 0.601; p < 0.01), although the differences between the regimens were significant for both groups.
Moreover, the ORR observed with U2 exceeded the response rate achieved in the control arm (83.3 % vs. 68.7 %; p < 0.001); this also applied to the proportions of patients with CR/CRi (5 % vs. 1 %). ORRs favored the novel regimen in both treatment-naïve and previously treated patients. In the population who had received prior BTK inhibition, those in the U2 arm fared comparatively better (ORR, 57 % vs. 25 %). Also, responses were durable, with 62 % still responding at 2 years. Disease control resulted in 93 %. Grade ≥ 3 AEs occurred more commonly with the novel combination (82 % vs. 66 %), which might be explained by the fact that median exposure was more than 4 times longer than in the control arm (21 vs. 5 months). The most common AEs observed in the experimental arm included diarrhea, nausea, and infusion-related reactions. At the grade 3/4 level, only neutropenia and diarrhea showed substantial rates (13 % and 12 %, respectively). While diarrhea occurred more frequently in the treatment-naïve population than in the previously treated patients, the opposite was true for neutropenia.
In their conclusion, the authors noted that UNITY-CLL is the first randomized trial of a PI3Kδ inhibitor in treatment-naïve CLL, thus establishing a new mechanism of action in this setting. The non-chemotherapy U2 regimen is highly active in CLL patients and is being explored as a backbone for triplets including combinations with venetoclax and BTK inhibitors.
Figure 4: Response rates according to iwCLL criteria observed in the BOVen study
U2 followed by venetoclax
Based on the observation that targeting of PI3K might prevent drug resistance to Bcl-2 inhibition [34, 35], a multicenter, phase I/II, dose-escalation trial investigated U2 in combination with venetoclax [36]. Patients with r/r CLL received a fixed dose of ublituximab (900 mg) and two dose levels of umbralisib (600 mg and 800 mg) for 3 cycles (induction/debulking period). This was followed by the consolidation period encompassing 9 cycles of venetoclax at the standard dose of 400 mg after a 5-week ramp up. The protocol was amended to add ublixitumab infusions on day 1 of cycle 4, 5 and 6, which coincided with the venetoclax treatment period. After the total 12-cycle treatment period, patients underwent full response assessment including MRD testing of their blood and marrow. Treatment was stopped in patients with uMRD, whereas those with detectable MRD continued on single-agent umbralisib. Forty-three and 39 patients were evaluable for safety and efficacy, respectively. The primary objective of the trial was the safety of the venetoclax addition after U2 induction.
U2 and venetoclax were shown to be well tolerated at the phase II doses. Only 7 % of patients discontinued the regimen prior to cycle 12. Also, U2 induction mitigated the TLS risk; after the induction phase, this risk showed an 81 % relative reduction. No patient developed clinical or laboratory TLS during the venetoclax ramp up. The regimen demonstrated encouraging efficacy in the patient cohort including those who were refractory to prior BTK inhibition (i.e., 52 % of the total population). All of the patients responded, and the CR rate at cycle 12 was 41 %. At that time, MRD was undetectable in 96 % and 77 % in the blood and bone marrow, respectively.
To date, only 1 patient has progressed 10 months after achieving uMRD in the blood and marrow and the discontinuation of therapy. Retreatment strategies are being investigated. The regimen is further explored in the setting of Richter’s transformation and in mantle cell lymphoma. Also, a phase II study assessing U2 plus venetoclax is ongoing in treatment-naïve and r/r CLL patients.
REFERENCES
- Burger JA et al., Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020; 34(3): 787-798
- Shanafelt TD et al., Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 2019; 381(1): 432-443
- Wierda WG et al., Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia/small lymphocyctic lymphoma: 1-year disease-free survival results from the MRD cohort of the phase 2 CAPTIVATE study. ASH 2020, abstract 123
- Al-Sawaf O et al., Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2020; 21(9): 1188-1200
- Scarfò L et al., Minimal residual disease-driven treatment intensification by sequential addition of ibrutinib to venetoclax in relapsed/refractory (r/r) chronic lymphocytic leukemia: results of the monotherapy and combination phases of the IMPROVE study. ASH 2020, abstract 2217
- Rogers KA et al., Three-year follow-up from a phase 2 study of combination obinutuzumab, ibrutinib, and venetoclax in chronic lymphocyctic leukemia. ASH 2020, abstract 1305
- Ghia P et al., Acalabrutinib vs idelalisib plus rituximab or bendamustine plus rituximab in relapsed/refractory chronic lymphocytic leukemia: ASCEND final results. ASH 2020, abstract 3140
- Woyach JA et al., Acalabrutinib in combination with venetoclax and obinutuzumab or rituximab in patients with treatment-naïve or relapsed/refractory chronic lymphocytic leukemia. ASH 2020, abstract 1312
- Davids MS et al., Updated safety and efficacy results from a phase 2 study of acalabrutinib, venetoclax and obinutuzumab (AVO) for frontline treatment of chronic lymphocytic leukemia (CLL). ASH 2020, abstract 2216
- Molica S et al., Comparison between time-limited, venetoclax-based and continuous Bruton tyrosine kinase inhibitor-based therapy in the upfront treatment of chronic lymphocytic leukemia (CLL): a systematic review. ASH 2020, abstract 3152
- Puiggros A et al., Genetic abnormalities in chronic lymphocytic leukemia: where we are and where we go. Biomed Res Int 2014; 2014: 435983
- Hallek M et al., Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376(9747): 1164-1174
- O’Brien S et al., Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol 2016; 17(10): 1409-1418
- Stilgenbauer S et al., Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol 2018; 36(19): 1973-1980
- Mato A et al., A clinical practice comparison of patients with CLL/SLL with and without del(17p) receiving first-line treatment with ibrutinib. ASH 2020, abstract 2498
- Guo Y et al., Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem 2019; 62(17): 7923-7940
- Tam CS et al., Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019; 134(11): 851-859
- Brown JR et al., Efficacy and safety of zanubrutinib in patients with treatment-naïve chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with del(17p): follow-up results from Arm C of the SEQUOIA (BGB-3111-304) trial. ASH 2020, abstract 1306
- Tam CS et al., Efficacy and safety of zanubrutinib in patients with treatment-naïve chronic lymphocytic (CLL) or small lymphocytic lymphoma (SLL) with del(17p): initial results from Arm C of the SEQUOIA (BGB-3111-304) trial. Blood 2019; 134(Supplement_1): 499
- Tam CS et al., A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 2020; 136(18): 2038-2050
- Song Y et al., Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton’s tyrosine kinase. Clin Cancer Res 2020; 26(16): 4216-4224
- Tam CS et al., Pooled analysis of safety data from monotherapy studies of the Bruton tyrosine kinase (BTK) inhibitor, zanubrutinib (BGB-3111), in B-cell malignancies. EHA 2019; abstract: PS1159
- Tam CS et al., Zanubrutinib in combination with venetoclax for patients with treatment-naïve chronic lymphocytic leukemia or small lymphocytic lymphoma and del(17p): Arm D of the SEQUOIA (BGB-3111-304) trial. ASH 2020, abstract 1318
- Soumerai JD et al., MRD-driven time-limited therapy with zanubrutinib, obinutuzumab, and venetoclax (BOVen) in previously untreated chronic lymphocytic leukemia. ASH 2020, abstract 1307
- Xu W et al., Earlier use of zanubrutinib monotherapy in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma associated with greater efficacy: a pooled analysis from 3 studies. ASH 2020, abstract 2229
- Xu W et al., Updates results from the phase II study of orelabrutinib monotherapy in Chinese patients with relapsed or refractory chronic lymphocytic leukemia/small cell lymphoma. ASH 2020, abstract 1320
- Mato AR et al., LOXO-305, a next generation, highly selective non-covalent BTK inhibitor in previously treated CLL/SLL: results from the phase 1/2 BRUIN study. ASH 2020, abstract 542
- Ghia P et al., Acalabrutinib versus idelalisib plus rituximab (IdR) or bendamustine plus rituximab (BR) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): ASCEND final results. Clin Lymphoma Myeloma Leukemia 2020; 20: S105-107 (abstract CLL-091)
- Lampson BL et al., Efficacy results of a phase 2 trial of first-line idelalisib plus ofatumumab in chronic lymphocytic leukemia. Blood Adv 2019; 3(7): 1167-1174
- Burris HA et al., Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol 2018; 19(4): 486-496
- Davids MS et al., An integrated safety analysis of the next generation PI3Kδ inhibitor umbralisib (TGR-1202) in patients with relapsed/refractory lymphoid malignancies. Blood 2017: 130(Suppl 1): 4037-4037
- Lunning M et al., Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2019; 134(21): 1811-1820
- Gribben JG et al., Phase 3 study of umbralisib combined with ublituximab vs obinutuzumab plus chlorambucil in patients with chronic lymphocyctic leukemia: results from UNITY-CLL. ASH 2020, abstract 543
- Cervantes-Gomez F et al., Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Cancer Res 2015; 21(16): 3705-3715
- Choudhary GS et al., MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis 2015; 6(1): e1593
- Barr PM et al., A phase 1/2 study of umbralisib, ublituximab and venetoclax in patients with relapsed or refractory chronic lymphocytic leukemia. ASH 2020, abstract 3137
© 2020 Springer-Verlag GmbH, Impressum