PD-1 inhibition and (Non-)Hodgkin lymphoma: promising outcomes in an emerging field
Nivolumab plus BV for MGZL treatment
Mediastinal gray zone lymphoma (MGZL) is an extremely rare type of Non-Hodgkin lymphoma with a predominance in young men [1]. This disease exhibits transitional features between nodular sclerosis classical Hodgkin lymphoma (cHL) and primary mediastinal B-cell lymphoma (PMBL) [2, 3]. However, compared to PMBL, survival of patients with MGZL is inferior after conventional chemotherapy [4]. The phase II Checkmate 436 trial has established high response rates for the anti-PD-L1 antibody nivolumab in combination with the antibody-drug conjugate brentuximab vedotin (BV) in adult patients with r/r PMBL [5]. Similarly, nivolumab plus BV conferred favorable results in patients with r/r cHL treated in the phase I/II setting [6].
In a separate cohort of the CheckMate 436 study, patients with r/r MGZL received nivolumab 240 mg plus BV 1.8 mg/kg 3-weekly until disease progression. The primary efficacy and safety analysis for the MGZL cohort (n = 10) was reported at ASH 2020 [7]. Refractory disease was present in 7 patients (70 %), and the median number of prior systemic cancer therapies was 2. None of the patients had undergone autologous stem cell transplantation (ASCT).
Findings that favor bridging
At database lock, all patients had discontinued treatment. The reasons included disease progression (n = 5), maximum clinical benefit (n = 3), allogeneic SCT (n = 1) and ASCT (n = 1). All of those with maximum clinical benefit had achieved complete remission and proceeded to allogeneic SCT. The ORR obtained in the total group was high at 70 %; complete and partial responses occurred in 50 % and 20 %, respectively. Also, time to CR was short (1.2–4.8 months). As the authors noted, these findings were consistent with the results reported for nivolumab plus BV in r/r PMBL and r/r cHL [5, 6, 8]. All patients who achieved CR were bridged to hematopoietic cell transplantation (i.e., 4 to allogeneic SCTs and 1 to ASCT).
Median OS had not been reached yet; at 6 months, 80 % of patients were alive. Duration of response and PFS could not be estimated reliably due to earlier censoring of patients who received subsequent therapies. The safety profile of the combination was tolerable and consistent with previous reports [5, 6, 8]. Cytopenia, paresthesia and peripheral sensory neuropathy occurred. No grade 4 AEs were observed. One patient was diagnosed with grade 3 febrile neutropenia, and another developed rash that was the only reported immune-mediated AE and resolved without systemic steroids. Based on these findings, the authors concluded that nivolumab plus BV represents a potential option for bridging to stem cell transplant in patients with chemotherapy-refractory MGZL.
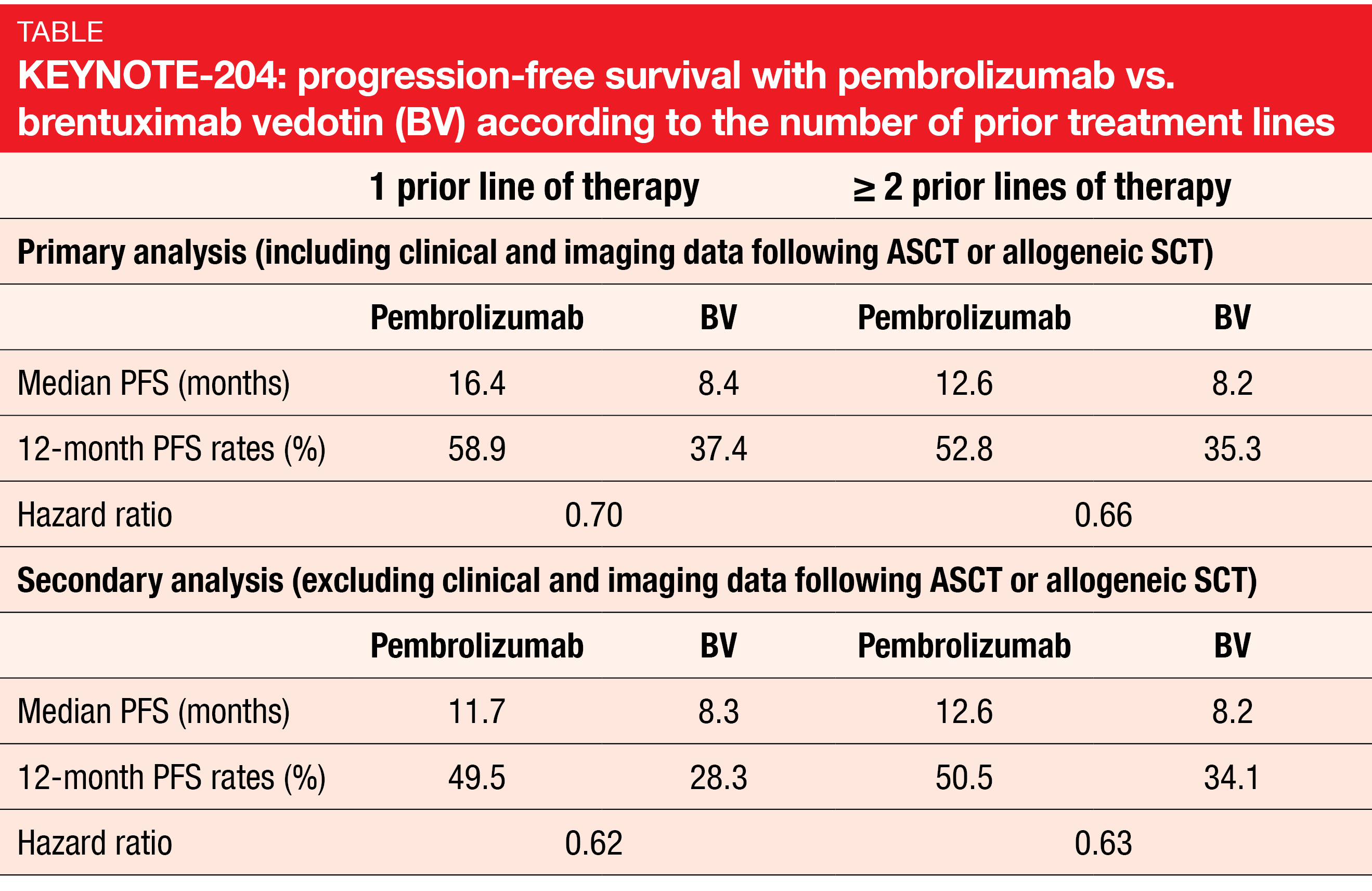
cHL: pembrolizumab vs. BV
Prognosis is poor for patients with relapsed/refractory cHL who have failed ASCT, have primary refractory disease or are ineligible for ASCT [9-13]. The open-label, international, randomized, phase III KEYNOTE-204 study tested the PD-1 inhibitor pembrolizumab as monotherapy (n = 151) against the antibody-drug conjugate brentuximab vedotin (BV; n = 153) in patients with r/r cHL. Treatment was administered for up to 35 cycles in both arms. The primary analysis presented at ASCO 2020 already showed a statistically significant and clinically meaningful PFS benefit for pembrolizumab compared to BV (13.2 vs. 8.3 months; HR, 0.65; p = 0.00271) [14]. At ASH 2020, Kuruvilla et al. presented a post-hoc exploratory analysis of the study that assessed the outcomes according to the number of prior lines of therapy [15]. Within the group of 55 patients who had previously been treated with one line, 27 and 28 had received pembrolizumab and BV, respectively. The cohort of patients after ≥ 2 treatment lines (n = 249) comprised 124 and 125 individuals randomized to the checkpoint inhibitor and BV, respectively.
Single-agent pembrolizumab was shown to improve clinical outcomes compared with BV regardless of the number of prior therapies. This held true irrespective of whether patients who went on to receive consolidative ASCT or allogeneic SCT were included in the data set or not (Table). Risk reductions ranged between 30 % and 38 %. Overall, these results were consistent with the primary analysis [14]. Likewise, ORRs were similar regardless of the treatment line. After one line, 66.7 % vs. 53.6 % of patients responded, and after ≥ 2 lines, this was true for 65.3 % vs. 54.4 %. Reponses lasted for a median of 20.7 vs. 14.1 months and 20.5 vs. 11.2 months, respectively. The safety profiles of both agents were generally similar across the subgroups. No unexpected safety signals occurred.
Also, subsequent ASCT was assessed by prior line of therapy. In the group of patients after one line, 25.9 % and 33.3 % of patients in the pembrolizumab and BV groups, respectively, underwent ASCT. For those with ≥ 2 pretreatment lines, this applied to 19.0 % and 20.0%, respectively. As the authors noted in their conclusion, these findings confirm that patients with r/r cHL after only one prior line of therapy who are ineligible for ASCT appear to benefit from pembrolizumab monotherapy.
Effect of TME on tislelizumab efficacy
Histologically, cHL has been shown to be characterized by low tumor cellularity (1–5 %) and a dominant tumor microenvironment (TME) composed of macrophages, T cells and other immune cells [16]. Limited efficacy of anti-PD-1 antibodies with a wild-type Fc region such as nivolumab and pembrolizumab might be due to binding to FcγR on macrophages, as this compromises the agents’ antitumor activity through activation of antibody-dependent macrophage-mediated killing of T effector cells [17, 18]. The anti-PD-1 antibody tislelizumab has been specifically engineered to minimize binding to FcγR on macrophages.
Preclinical data showed that in macrophage- and T-cell–enriched conditions, tislelizumab did not induce antibody-dependent cellular phagocytosis (ADCP), and thus its anti-tumor activity was not compromised [19]. A pivotal phase II trial investigating tislelizumab in Chinese patients with cHL that had failed or were no candidates for high-dose chemotherapy/ASCT revealed an ORR of 87.1 %, with a CR rate of 62.9 % [20]. The study reported at ASH 2020 explored whether FcγR expression on macrophages in the cHL TME impacts the efficacy of tislelizumab [21]. Moreover, additional biomarkers associated with CRs were assessed. Multiplex immunohistochemistry (mIHC) and gene expression profiling (GEP) were used to analyze samples from 70 patients included the BGB-A317-203 trial. Among these, 41 and 36 were mIHC- and GEP-evaluable, respectively.
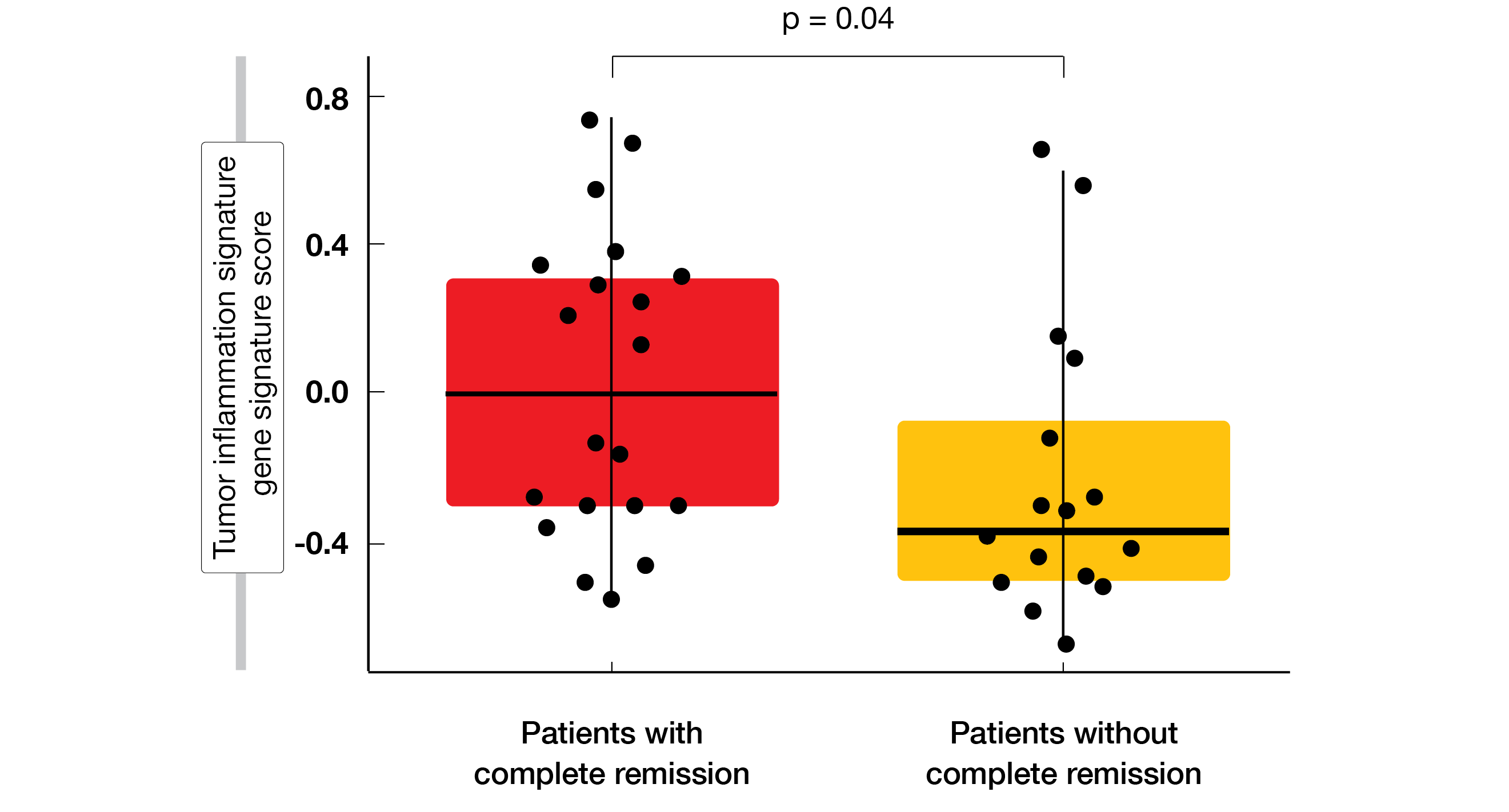
In accordance with the characteristics of tislelizumab, the agent demonstrated high CR rates regardless of FcγR expression levels on macrophages in the CD8+ T-cell–high TME where ADCP-induced effector T-cell clearance is more likely. The CR rates were 86.6 % vs. 85.7 % for high and low numbers of FcγR1+ macrophages, respectively. Multiple biomarkers were identified that correlated with complete responses to tislelizumab treatment. Higher CD8+ T-cell infiltration according to mIHC and tumor inflammation signature gene signatures in the TME by GEP corresponded with higher CR rates (Figure). Moreover, mixed cellularity and nodal sclerosis histological subtypes showed specific gene signatures associated with complete response.
Figure: Significantly increased inflammation of the tumor microenvironment in patients who developed complete response on tislelizumab treatment
REFERENCES
- Quintanilla-Martinez L, Fend F. Mediastinal gray zone lymphoma. Haematologica 2011; 96(4): 496-499
- Eberle FC et al., Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin’s lymphoma and primary mediastinal large B-cell lymphoma. Haematologica 2011; 96(4): 558-566
- Melani C et al., PD-1 blockade in mediastinal gray-zone lymphoma. N Engl J Med 2017; 377(1): 89-91
- Wilson WH et al., A prospective study of mediastinal gray-zone lymphoma. Blood 2014; 124(10): 1563-1569
- Zinzani PL et al., Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-cell lymphoma: efficacy and safety from the phase II CheckMate 436 Study. J Clin Oncol 2019; 37(33): 3081-3089
- Moskowitz AJ et al., Brentuximab vedotin and nivolumab for relapsed or refractory classic Hodgkin Lymphoma: long-term follow-up results from the single-arm phase 1/2 study. Blood 2019; 134 (Supplement_1): 238
- Santoro A et al., Nivolumab combined with brentuximab vedotin for relapsed/refractory mediastinal gray zone lymphoma: primary efficacy and safety analysis of the phase 2 CheckMate 436 study. ASH 2020, abstract 2045
- Herrara AF et al., Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018; 131(11): 1183-1194
- Fermé C et al., Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy: results of the Groupe d’Etudes des Lymphomes de l’Adulte H89 Trial. J Clin Oncol 2002; 20(2): 467-475
- Goodman KA et al., Long-term effects of high-dose chemotherapy and radiation for relapsed and refractory Hodgkin’s lymphoma. J Clin Oncol 2008; 26(32): 5240-5247
- Santoro A et al., Five-year results of the BEGEV salvage regimen in relapsed/refractory classical Hodgkin lymphoma. Blood Adv 2020; 4(1): 136-140
- Puig N et al., Different response to salvage chemotherapy but similar post-transplant outcomes in patients with relapsed and refractory Hodgkin’s lymphoma. Haematologica 2010; 95(9): 1496-1502
- Younes A et al., Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 2012; 30(18): 2183-2189
- Kuruvilla J et al., KEYNOTE-204: Randomized, open-label, phase III study of pembrolizumab (pembro) versus brentuximab vedotin (BV) in relapsed or refractory classic Hodgkin lymphoma (R/R cHL). J Clin Oncol 2020; 38(suppl), 8005
- Kuruvilla J et al., Effect of pembrolizumab monotherapy compared with brentuximab vedotin on patients with relapsed/refractory classical Hodgkin lymphoma by prior lines of therapy: an exploratory analysis of the randomized phase 3 KEYNOTE-204 study. ASH 2020, abstract 1158
- Aoki T et al., Single-cell transcriptome analysis reveals disease-defining T-cell subsets in the tumor microenvironment of classic Hodgkin lymphoma. Cancer Discov 2020; 10(3): 406-421
- Dahan R et al., FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell 2015; 28(3): 285-295
- Arlauckas SP et al., In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med 2017; 9(389): eeal3604
- Zhang T et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018; 67(7): 1079-1090
- Song Y, et al., Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia 2020; 34(2): 533-542
- Song Y et al., Tumor microenvironment associated with complete response to tislelizumab monotherapy in relapsed/refractory classical Hodgkin lymphoma reveals a potentially different mechanism of action. ASH 2020, abstract 1116
© 2020 Springer-Verlag GmbH, Impressum