What is new in Waldenström’s macroglobulinemia?
Constitutive activation of the Bruton’s tyrosine kinase (BTK) pathway has been shown to induce malignant cell survival in patients with Waldenström’s macroglobulinemia (WM) [1, 2]. The disease is based on the accumulation of IgM-secreting clonal lymphoplasmacytic cells in the bone marrow and extramedullary sites [3]. MYD88L265P mutations (> 90 % of cases) and CXCR4WHIM-like mutations (approximately 27 % of cases) have been established as the pathologic hallmarks of WM [4-6].
iNNOVATE: ibrutinib plus rituximab
BTK inhibition with ibrutinib has dramatically changed the treatment landscape in WM. The phase III iNNOVATE study compared ibrutinib plus rituximab with placebo plus rituximab in WM patients who were either treatment-naïve or pretreated; the latter had to be rituximab-sensitive (i.e., not refractory to the last prior rituximab-based therapy and no treatment with rituximab within the last 12 months before the first study dose). Each arm included 75 individuals. After a median follow-up of 26.5 months, the primary analysis of iNNOVATE demonstrated superiority of the combination over rituximab monotherapy [7]. These data formed the basis for the approval of ibrutinib plus rituximab in the United States and Europe.
At ASH 2020, Buske et al. reported the final analysis of the study after an overall follow-up of 63 months [8]. The protocol permitted cross-over to single-agent ibrutinib after disease progression in the control arm. Indeed, 35 (47 %) of these patients crossed over. After study closure, 68 (45 %) remained on ibrutinib, as 32 enrolled in a treatment extension program and 36 continued to receive ibrutinib in a commercial setting.
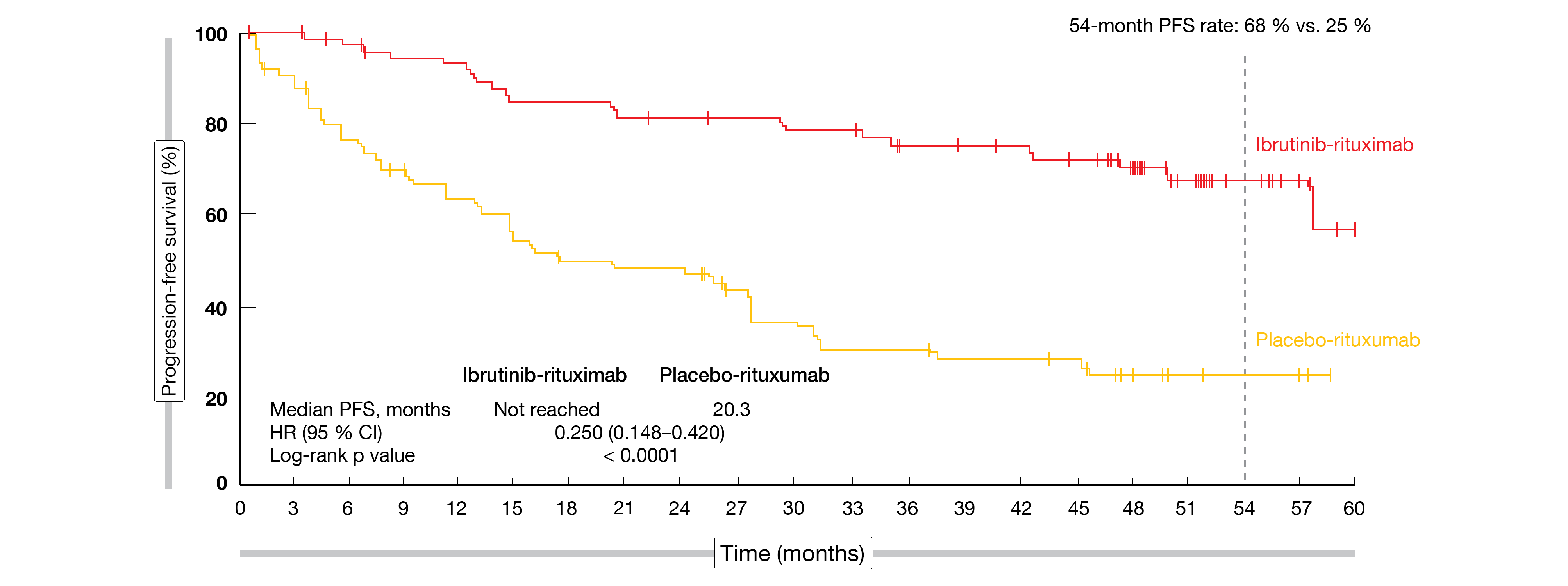
Even 5 years after the initiation of the trial, median progression-free survival (PFS) had not been reached in the experimental arm. Compared to the median PFS of 20.3 months observed with placebo plus rituximab, this translated into a 75 % risk reduction (HR, 0.25; p < 0.0001; Figure). At 54 months, PFS rates were 68 % vs. 25 %. In both arms, the PFS benefit did not depend on the genotype (i.e., any combinations of MYD88L265P or MYD88 wildtype and CXCR4WHIM or CXCR4 wildtype). Moreover, ibrutinib plus rituximab improved PFS irrespective of the prior treatment status; in both pretreated and not pretreated patients, PFS was superior in all prespecified subgroups that received the combination.
Figure: Progression-free survival benefit with ibrutinib plus rituximab vs. placebo plus rituximab
Rapid and sustained improvements
Responses occurred early on in the experimental arm, with median time to major response amounting to 3 months vs. 6 months in the control arm. Overall, the major response rates were 76 % vs. 31 % for the two arms. The proportion of patients experiencing very good partial responses increased over time with ibrutinib plus rituximab. Again, responses were largely independent of the genotype and prior treatment status.
IgM levels decreased rapidly during the first year of treatment. Maximum changes were -33.5 g/L for the combination at 56 months and -26.9 g/L for rituximab monotherapy at 57 months. Also, a larger fraction of patients treated in the experimental arm experienced sustained hemoglobin improvement, which was defined as increases of ≥ 20 g/L (or ≥ 5 g/L if baseline levels were ≤ 110 g/L) that persisted for ≥ 8 weeks without the need of blood transfusions or growth factors. This was the case for both the total group (77 % vs. 43 %; p < 0.0001) and the group with low baseline hemoglobin levels (95 % vs. 56 %; p < 0.0001). Median overall survival (OS) had not been reached yet in either treatment arm. The 54-month OS rates were 86 % vs. 84 %.
After 63 months of follow-up, the combination maintained a manageable safety profile, and no new safety signals emerged. The incidences of the main adverse events (AEs) including diarrhea, arthralgia and hypertension decreased over time. In year 4–5, AE-related ibrutinib dose reductions and treatment discontinuation became necessary in 5 % each. Eighty-eight percent of AEs that led to ibrutinib dose reductions subsequently resolved. In their conclusion, the authors noted that ibrutinib plus rituximab showed ongoing superiority across different clinical outcomes in patients with Waldenström’s macroglobulinemia.
Phase II data for zanubrutinib
The selective, irreversible next-generation BTK inhibitor zanubrutinib has been designed to maximize BTK occupancy and minimize off-target inhibition of other kinases [9-11]. A pivotal, single-arm, open-label, multicenter phase II trial evaluated zanubrutinib 160 mg twice daily until progression in 44 Chinese patients with relapsed and refractory WM [12]. They had received ≥ 1 prior line of standard chemotherapy-containing treatment and had failed to achieve at least minor response or had progressed after response to the most recent regimen. The major response rate (MMR) was defined as the primary endpoint; this included complete, partial, and very good partial responses as assessed by an independent review committee. Seventy-five percent of patients had intermediate or high risk according to the WM prognostic score. A median of 2 prior systemic regimens had been administered. The study also enrolled MYD88-wildtype patients, who made up 15.9 % of the total population. Anemia (hemoglobin ≤ 110 g/L) was present at baseline in 75 %.
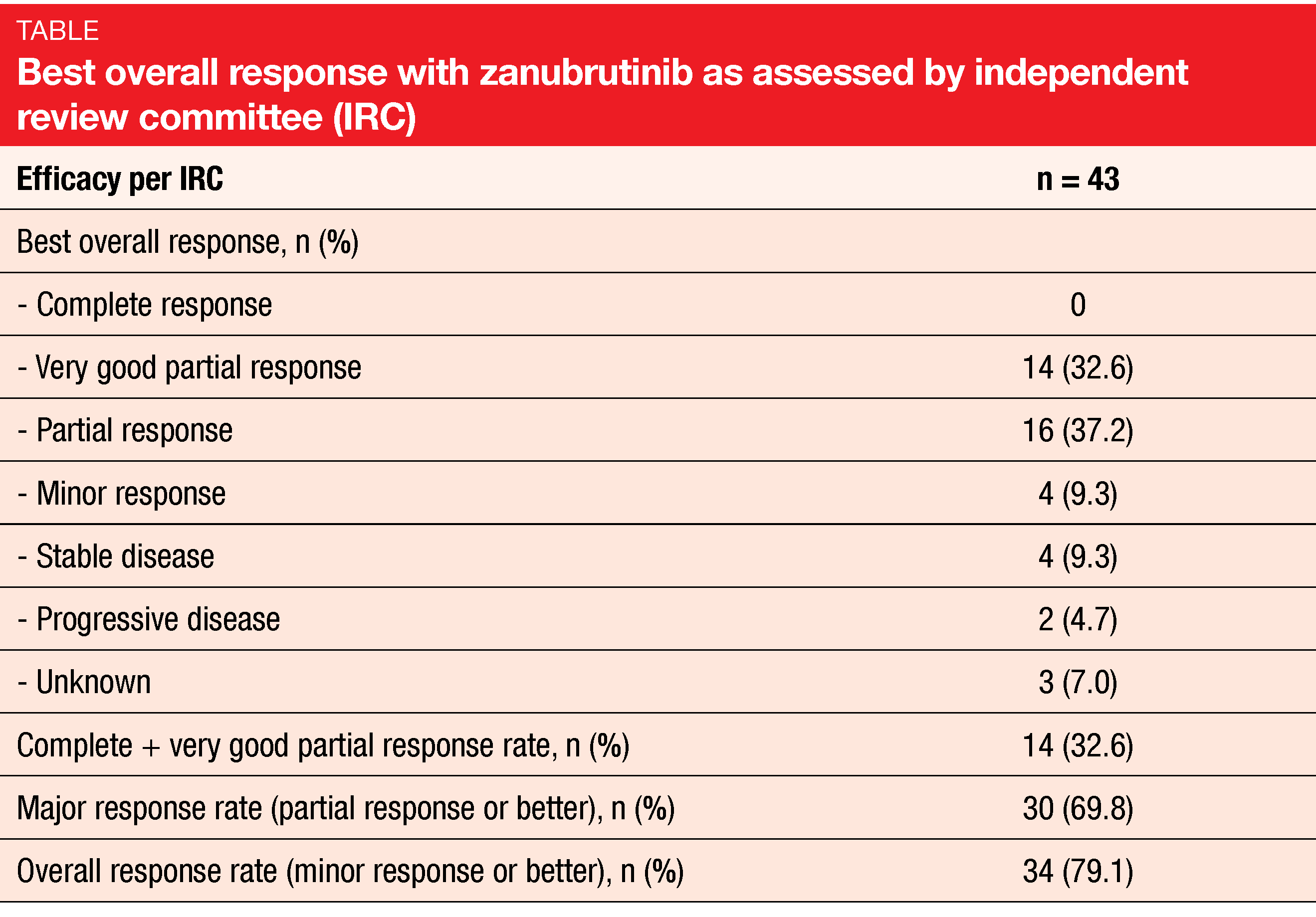
After a median follow-up of 18.6 months, zanubrutinib demonstrated pronounced and durable efficacy. Almost 70 % of patients developed MMR, with 32.6 % experiencing very good partial remission (Table). Responses were achieved quickly; median time to overall response was 2.76 months. Neither median PFS nor the median duration of major response had been reached yet. At 12 months, 78.3 % of patients were progression-free, and 88.1 % showed ongoing major responses. The MMR benefit of zanubrutinib was generally consistent across subgroups. Treatment-related AEs mainly included neutropenia, thrombocytopenia, infections, and diarrhea. The safety and tolerability profiles of zanubrutinib corresponded to those reported previously in WM patients. Study drug discontinuation was required in 11.4 %.
These results have been submitted to the Chinese National Medical Product Administration for approval of zanubrutinib in patients with WM. The European approval in this indication based on the phase III ASPEN trial is expected for summer 2021 [13, 14].
Chinese real-world observation
A large, multicenter, retrospective study conducted in China assessed the clinical presentation, frontline treatment, outcome and prognosis of WM in patients diagnosed between January 2003 and December 2019 at 35 tertiary hospitals in 22 provinces [15]. Overall, 1,141 patients with a median age of 63 years were enrolled. Forty percent were older than 65 years. The male-to-female ratio was 2.7:1. According to the revised International Prognostic Scoring System (rIPSS), most of the patients had low (n = 342) and intermediate (n = 325) risk.
Documented treatment information was available for 734 patients. Due to the heterogeneous clinical presentations and the rarity of the disease, frontline treatment choices showed a considerable variety. Monotherapies such as chlorambucil, ibrutinib and rituximab represented 10.2 % of the total. Chemoimmunotherapy (e.g., rituximab/dexamethasone/cyclophosphamide, rituximab/prednisone/cyclophosphamide, R-COP, R-CHOP, rituximab/fludarabine/cyclophosphamide) accounted for 36.0 %. In 53.8 %, other combinations were used, with bortezomib-based regimens ranging first, followed by fludarabine/cyclophosphamide, CHOP and immunomodulatory agents plus dexamethasone.
After a median follow-up of 32 months, the estimated 3-year OS rate was 83 %. Most of the established prognostic factors according to rIPSS indicated worse prognosis in this population. These included LDH levels ≥ 250 IU/L, albumin levels < 3.5 g/dL, β-2 microglobulin ≥ 4 mg/L, and age > 65 years. The median OS of patients aged ≤ 65 years had not been reached yet, while this was 132 months and 61 months for those aged 66-75 years and > 75 years, respectively. Also, platelet counts ≤ 100 x 109/L constituted a prognostic factor.
REFERENCES
- Rickert RC, New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol 2013; 13(8): 578-591
- Argyropoulos KV et al., Clonal B cells in Waldenström’s macroglobulinemia exhibit functional features of chronic active B-cell receptor signaling. Leukemia 2016; 30(5): 1116-1125
- Janz S, Waldenström macroglobulinemia: clinical and immunological aspects, natural history, cell of origin, and emerging mouse models. ISRN Hematol 2013; 2013: 815325
- Treon SP et al., MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med 2012; 367: 826-833
- Hunter ZR et al., The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014; 123: 1637-1646
- Roccaro AM et al., C1013G/CXCR4 acts as a driver mutation of tumor progression and modulator of drug resistance in lymphoplasmacytic lymphoma. Blood 2014; 123: 4120-4131
- Dimopoulos MA et al., Phase 3 trial of ibrutinib plus rituximab in Waldenström’s macroglobulinemia. N Engl J Med 2018; 378(25): 2399-2410
- Buske C et al., Five-year follow-up of ibrutinib plus rituximab vs placebo plus rituximab for Waldenström’s macroglobulinemia: final analysis from the randomized phase 3 iNNOVATETM study. ASH 2020, abstract 336
- Guo Y et al., Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of bruton’s tyrosine kinase. J Med Chem 2019; 62(17): 7923-7940
- Tam CS et al., Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019; 134(11): 851-859
- Mu S et al., Effect of rifampin and itraconazole on the pharmacokinetics of zanubrutinib (a Bruton’s tyrosine kinase inhibitor) in Asian and non-Asian healthy subjects. Cancer Chemother Pharmacol 2020; 85: 391-399
- An G et al., Safety and efficacy of the Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) in patients with Waldenström macroglobulinemia from a phase 2 trial. ASH 2020, abstract 2940
- Dimopoulos M et al., ASPEN: Results of phase 3 randomized trial of zanubrutinib versus ibrutinib for patients with Waldenström macroglobulinemia. EHA 2020, abstract S225
- Dimopoulos M et al., Updated results of the ASPEN trial from a cohort of patients with MYD88 wild-type Waldenström macroglobulinemia. EHA 2020, abstract EP1180
- Cao XX et al., Treatment and outcome patterns of patients with Waldenström’s macroglobulinemia: a large, multicenter retrospective review in China. ASH 2020, abstract 2053
© 2020 Springer-Verlag GmbH, Impressum