Determining first-line CLL/SLL treatment strategies with optimized efficacy and safety
GAIA: FCR compared to targeted regimens
The international, randomized, phase III GAIA/CLL13 study was conducted to identify the optimal time-limited first-line treatment regimen for fit patients with chronic lymphocytic leukemia (CLL). Standard chemoimmunotherapy (CIT) consisting of fludarabine, cyclophosphamide and rituximab (FCR; patients ≤ 65 years) or bendamustin plus rituximab (BR; patients > 65 years) was compared to venetoclax-based, limited-duration strategies. Patients with CIRS ≤ 6 and normal creatinine clearance (> 70 mL/min), in whom TP53 mutation and del(17) had been excluded, participated in the trial. The comparator regimens consisted of rituximab plus venetoclax (RVe), obinutuzumab plus venetoclax (GVe), and obinutuzumab plus ibrutinib and venetoclax (GIVe). Each arm contained approximately 230 patients. Co-primary endpoints of the study included the undetectable minimal residual disease (uMRD) < 10-4 rate at month 15 in peripheral blood by 4-color flow, and progression-free survival (PFS). At ASH 2021, Eichhorst et at al. presented the results for the uMRD outcome [1]. The PFS interim analysis had been postponed to early 2022 due to the low number of events.
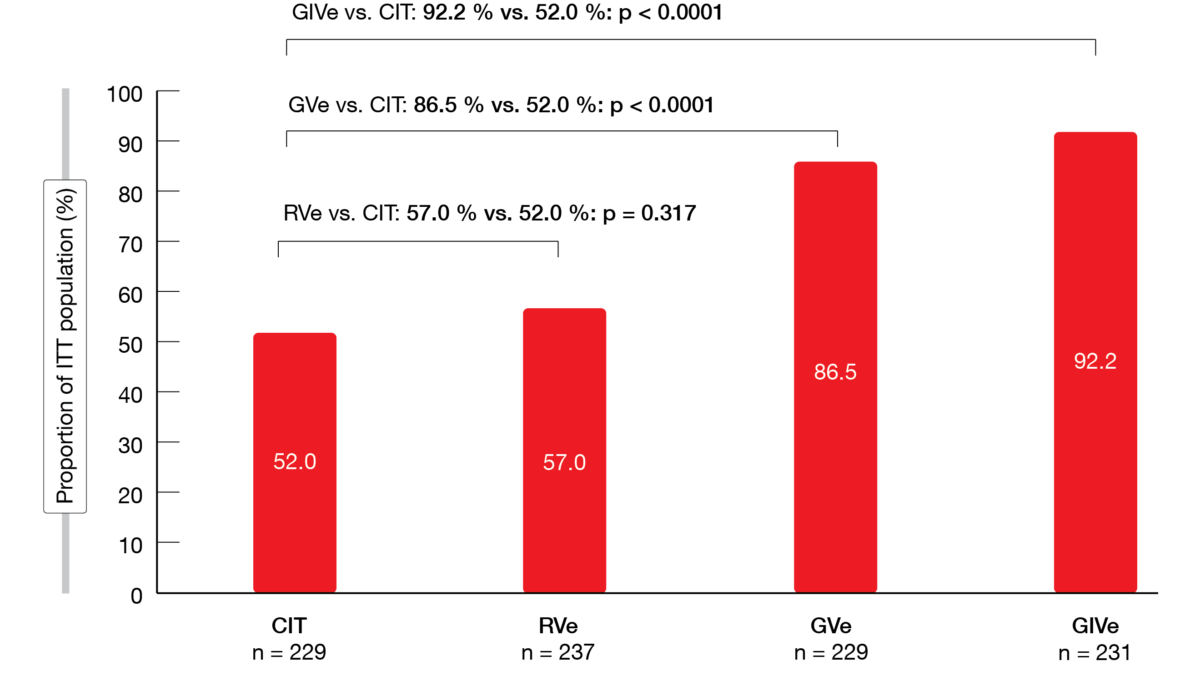
After a median follow-up of 27.9 months, the uMRD rate was significantly higher in the GVe and GIVe arms compared to CIT (86.5 % and 92.2 %, respectively, vs. 52.0 %; p < 0.0001 each; Figure 1). For the RVe regimen, this was 57 %, which meant that RVe was not superior to CIT. Overall response rates according to iwCLL were relatively high in all arms, although rates of complete responses with or without complete hematological recovery (CR/CRi) in the venetoclax-based arms exceeded those in the CIT arm (49.4 %, 56.8 % and 61.9 % for RVe, GVe and GIVe, respectively, vs. 31 % for CIT).
Neutropenia was the most frequent grade ≥ 3 adverse event (AE) across all arms. While febrile neutropenia occurred more frequently with CIT (11.1 %), severe infections were seen most commonly with GIVe (22.1 %) and CIT (19.9 %). The incidence of grade ≥ 3 tumor lysis syndrome ranged from 4.2 % (CIT) to 10.1 % (RVe). Grade ≥ 3 bleeding events and atrial fibrillation occurred in approximately 2 % in the GIVe arm and were very rare in the other arms. Twelve patients died due to AEs during treatment and until day 84 after the end of treatment; this was mostly due to infections. Fifteen deaths during the follow-up period were mostly due to secondary neoplasia. The rate of treatment discontinuation was low in all experimental arms.
Figure 1: GAIA trial: uMRD < 10-4 rates in the peripheral blood at month 15 with chemoimmunotherapy (CIT) vs. venetoclax-based, limited-duration regimens
FCR plus ibrutinib in fit patients
Ibrutinib-based treatment has been shown to prolong survival compared to FCR in CLL patients with unmutated IGHV [2]. It was hypothesized that combining ibrutinib with FCR (iFCR) as initial therapy would lead to high CR rates with uMRD in the bone marrow in a broad population of younger, fit CLL patients. The phase II study launched in 2014 enrolled a total of 85 patients at 7 US sites with a median age of 55 years. After a 1-week lead-in with ibrutinib alone, iFCR was administered for up to 6 cycles, followed by ibrutinib maintenance for 2 years. Patients who achieved uMRD at the end of this period discontinued therapy, while those who did not continued treatment until progression. Retreatment with ibrutinib was allowed in patients who relapsed. The primary endpoint of the trial was the CR rate with uMRD in the bone marrow 2 months after iFCR completion.
According to the first analysis published in 2019, the primary endpoint was met after a median follow-up of 16.5 months, with 33 % of patients achieving CR as defined above [3]. The best uMRD rate in the bone marrow by intent-to-treat (ITT) was 84 % at that time, which was higher than results obtained with any prior CIT or novel-agent–based regimen for initial CLL therapy.
Deepening of remissions
The updated efficacy analysis reported at ASH 2021 showed that the best CR rate with uMRD in the bone marrow by ITT had increased to 55 % with ibrutinib maintenance [4]. Complete remissions had deepened with ibrutinib maintenance from 34 % 2 months after FCR to 81 % as best rate; for patients with mutated IGHV, increases had occurred from 41 % to 88 %, and for those with unmutated IGHV, from 28 % to 76 %. The best rate of uMRD in the bone marrow by ITT remained at 84 %. In the 81 patients with TP53 wildtype, the best MRD-negative rate by ITT in the bone marrow amounted to 91 %.
Two years after the end of treatment, 86.5 % of patients achieved MRD 10-4 in the peripheral blood by flow cytometry; by NGS, this was 91.0 %. Dynamic BH3 profiling suggested that increased CLL cell BCL-2 dependence after 1 week of ibrutinib treatment might predict deeper clinical responses. PFS and overall survival were promising, with rates of 97 % and 99 %, respectively, at a median follow-up of 40.3 months. All of the few patients who experienced recurrence responded to re-treatment with ibrutinib monotherapy.
Compared to the previous report, the updated safety analysis showed increases in the rates of grade 3/4 neutropenia (from 35 % to 40 %), febrile neutropenia (from 9 % to 12 %), and atrial fibrillation (from 3.5 % to 8 %). Grade 3/4 thrombocytopenia and anemia remained unchanged (32 % and 11 %, respectively). No Richter’s syndrome has been observed to date. Overall, the safety profile was consistent with individual toxicities of ibrutinib and FCR. The authors concluded that iFCR warrants exploration in comparative studies in a broad population of younger, fit CLL patients with intact TP53 who desire functional cure with time-limited treatment approaches.
FLAIR: FCR vs. ibrutinib/rituximab
The frontline comparison of FCR (n = 385) with ibrutinib/rituximab (IR; n = 386) in patients considered fit for FCR was at the heart of the randomized NRCI FLAIR trial. Rituximab was administered for 6 cycles, while ibrutinib was taken orally for a maximum of 6 years or until sustained MRD negativity. The MRD status was assessed every 6 months based on blood.
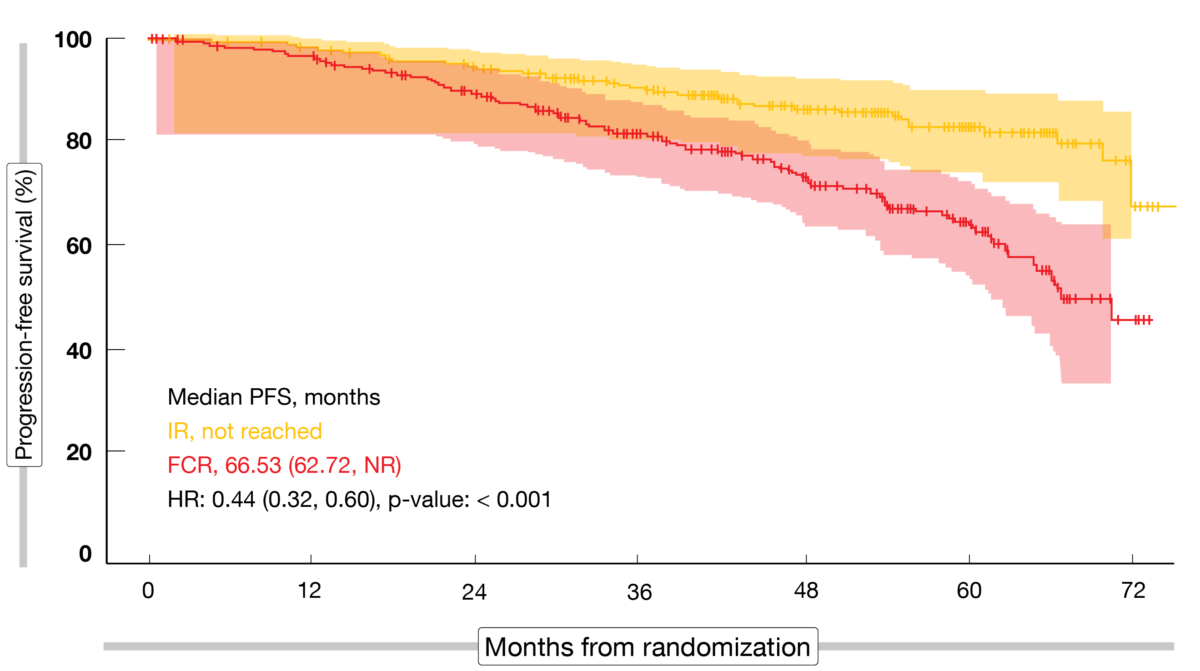
After a median follow-up of 52.7 months, the primary endpoint of the FLAIR trial was met: IR was superior compared to FCR regarding PFS (not reached vs. 66.53 months; HR, 0.44; p < 0.001; Figure 2) [5]. PFS was significantly prolonged in the experimental arm in patients with unmutated IGHV (HR, 0.40; p < 0.001), whereas a non-significant improvement resulted in IGHV-mutated disease (HR, 0.68; p = 0.197). Moreover, significant PFS advantages emerged with IR in patients harboring 11q deletion and normal karyotype but not in those with trisomy 12 and 13q deletion. Three months after the end of treatment, greater proportions of FCR-treated patients showed CR (60.5 % vs. 21.0 %) and MRD negativity in the bone marrow (55.3 % vs. 3.9 %). Overall survival had not been reached yet in either arm, with superimposable curves (HR, 1.01); however, it must be noted that almost all patients relapsing after FCR received either ibrutinib or venetoclax plus rituximab.
Among the most frequent AEs reported within one year of randomization, anemia, nausea and decreased white blood cell counts were more common with FCR than with IR, as well as infusion-related reactions and grade ≥ 3 decreases of white blood cells, while diarrhea was substantially more common with IR. Twenty-nine and 30 patients died in the FCR and IR arms, respectively. Deaths in the FCR arm were predominantly due to secondary hematological malignancies, Richter’s transformation, and infections. Those in the IR arm, on the other hand, were mostly related to cardiac causes and non-hematological malignancies. Sudden unexplained death or cardiac death occurred more commonly with IR than with FCR (8 vs. 2); most of these patients (7 of 8) had hypertension or a prior history of cardiac disorder requiring therapy at trial entry. The authors noted that FLAIR is not an outlier for sudden unexplained or cardiac deaths in ibrutinib-containing arms and is consistent with other phase III trials assessing ibrutinib-based regimens in CLL.
Figure 2: Primary endpoint of the FLAIR study: progression-free survival improvement with ibrutinib/rituximab vs. FCR
Arms A and B of the SEQUOIA trial
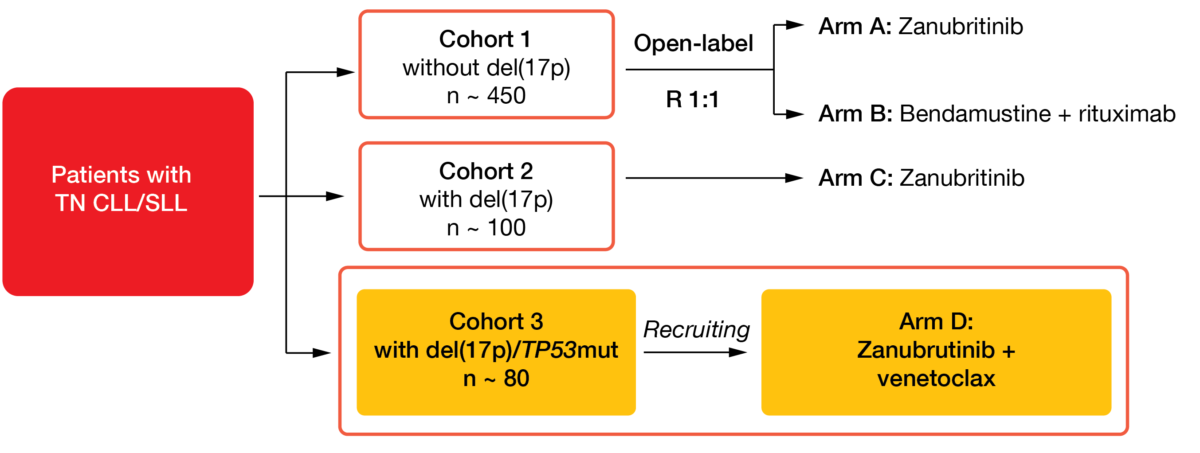
Zanubrutinib is a highly selective second-generation BTK inhibitor designed to maximize BTK occupancy and minimize off-target effects [6, 7]. The phase III SEQUOIA trial assessed zanubrutinib as monotherapy and combination partner in treatment-naïve patients with CLL or small lymphocytic lymphoma (SLL) ≥ 65 years of age or unsuitable for FCR therapy (Figure 3). Results obtained for Arm C of the study already suggested efficacy and tolerability of single-agent zanubrutinib in patients with del(17p) [8, 9]. Arms A and B of the SEQUOIA study compared zanubrutinib to BR in patients who did not have del(17p). The analysis of this cohort presented by Tam et al. at ASH 2021 included 241 and 238 patients in the experimental and control arms, respectively [10].
Zanubrutinib was shown to induce a PFS benefit over BR according to independent review, with 24-month rates of 85.5 % vs. 69.5 % (p < 0.0001). This translated into a 58 % risk reduction (HR, 0.42) and was comparable to the PFS achieved with zanubrutinib monotherapy in Arm C of the SEQUOIA study that contained patients with del(17p). The novel BTK inhibitor performed better across all of the important prognostic subgroups, which also applied to high-risk groups with del(11q) and unmutated IGHV. Patients with unmutated IGHV achieved a 76 % reduction in the risk of progression or death on zanubrutinib treatment compared to BR (HR, 0.24; p < 0.001). For those with mutated IGHV status, this difference had not become significant yet (HR, 0.67; p = 0.186).
Consistent with other studies, zanubrutinib appeared to be well tolerated. Treatment with the novel BTK inhibitor, as compared to BR, was associated with lower grade ≥ 3 AEs (52.5 % vs. 79.7 %) and AEs leading to dose reduction (7.5 % vs. 37.4 %), dose interruption/delay (46.3 % vs. 67.8%), or treatment discontinuation (8.3 % vs. 13.7 %). Notably, cytopenias were observed less often, whereas bleeding occurred more commonly (45.0 % vs. 11.0 %). The rate of atrial fibrillation, which is a commonly observed AE of the first-in-class BTK inhibitor ibrutinib, was equally low in both arms (3.3 % vs. 2.6 %).
Figure 3: Design of the SEQUOIA trial assessing zanubrutinib as monotherapy and combination partner in patients with treatment-naïve CLL/SLL
Zanubrutinib/venetoclax: Arm D
Moreover, early results for Arm D of the SEQUOIA study were reported at ASH 2021 [11]. In this arm, zanubrutinib for ≥ 27 cycles is being evaluated together with venetoclax for 12-24 cycles in patients with del(17) and/or TP53 variants. Both drugs can be discontinued upon confirmed uMRD; for zanubrutinib, this is the case from cycle 28, and for venetoclax, from cycle 16. The analysis included 49 and 36 individuals in the safety and efficacy populations, respectively.
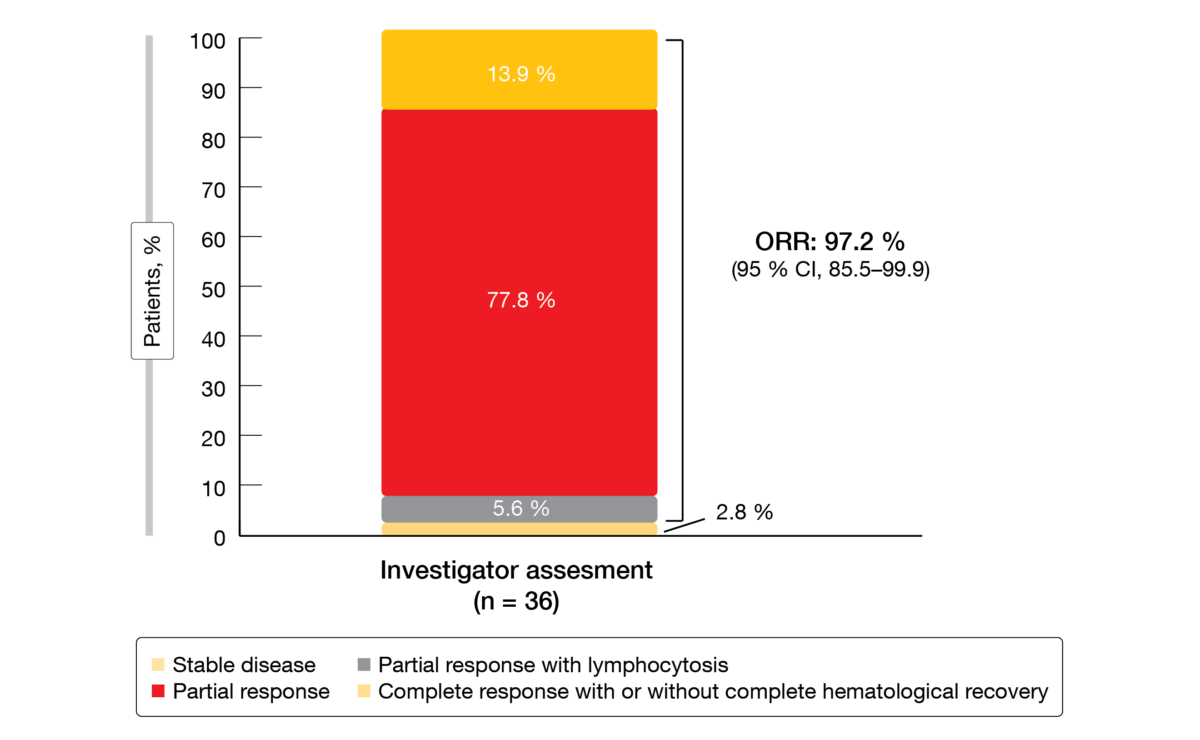
After a median follow-up of 12 months, zanubrutinib plus venetoclax gave rise to a high overall response rate of 97.2 % in this high-risk population (Figure 4). Almost 14 % obtained CR/CRi; by 36 months, 4 patients had developed uMRD. The authors noted that responses appeared to deepen with longer treatment, as indicated by the achievement of CR/CRi and uMRD. After the 12-month follow-up, only one patient had developed progression, and one fatality occurred due to lung carcinoma prior to the initiation of venetoclax treatment. In the remaining group, treatment was ongoing. Fourteen patients had been treated with the combination for at least 12 months at the time of the analysis.
The combination was well tolerated, with no reported cases of clinical tumor lysis syndrome and relatively low incidences of neutropenia (all grades, 20.6 %), diarrhea (14.7), and nausea (14.7 %). Patients on combination treatment experienced grade ≥ 3 AEs in 38.2 %; in this group, AEs necessitated dose interruptions in 29.4 % but did not give rise to dose reductions or treatment discontinuation. No fatal AEs occurred. According to the investigators’ conclusion, a more mature follow-up is needed to fully assess the depth of response and the safety of zanubrutinib plus venetoclax in this high-risk population.
Figure 4: Responses obtained with zanubrutinib plus venetoclax in Arm D of the SEQUOIA study
Long-term results for ibrutinib-based strategies
Various ibrutinib-containing regimens are being compared with BR in patients aged ≥ 65 years in the randomized phase III A041202 trial. Arm 1 is receiving BR (n = 183), Arm 2 ibrutinib alone (n = 182), and Arm 3 IR (n = 182). According to the primary analysis published in 2018, median PFS was significantly longer in Arms 2 and 3 than in Arm 1 (HRs, 0.39 and 0.38, respectively; p < 0.001 each), while there was no significant difference between Arms 2 and 3 [12]. Updated findings were presented after the third planned interim analysis of Arms 2 and 3 vs. Arm 1, as well as the second planned interim analysis of Arm 3 vs. Arm 2 [13].
Pairwise comparisons after a median follow-up of 55 months revealed 64 % reductions in the risk of progression or death for both ibrutinib vs. BR and IR vs. BR (p < 0.0001 each). For IR vs. ibrutinib, the PFS difference was not significant (HR, 0.99; p = 0.96). Ibrutinib-based treatment performed better than BR in terms of PFS in all patient subgroups. Multivariable models were conducted to determine prognostic factors associated with PFS. When testing for interaction effects between treatment groups, it was shown that the treatment effect was significantly different for age, TP53 abnormalities and Zap-70 methylation. Although the protective effect of ibrutinib was evident across subgroups, ibrutinib-based regimens appeared to be even more protective for younger patients and those harboring genomic abnormalities with the highest risk. Ibrutinib-treated patients experienced improved PFS compared to those receiving BR independent of the number of karyotype abnormalities. Patients with TP53 aberrations derived a significant PFS advantage when treated with ibrutinib-based therapies (HR, 0.07); the same applied to Zap-70 methylation < 20 % (HR, 0.18).
With respect to toxicity, it was noted that atrial fibrillation and hypertension were more common in the groups receiving ibrutinib-based therapies than in the BR-treated cohort and increased over time on therapy. However, they did not appear to outweigh the superior efficacy of treatment in this particular setting.
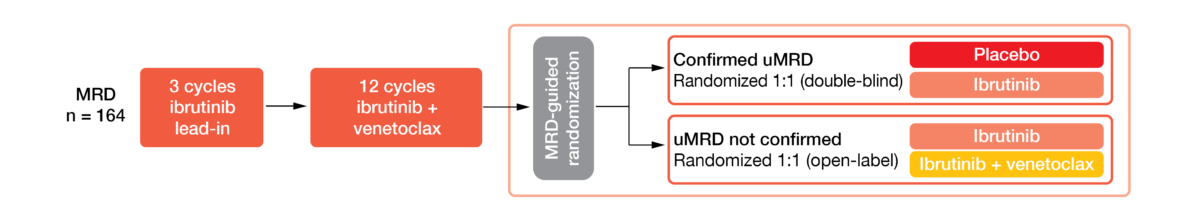
MRD cohort of CAPTIVATE
The international phase II CAPTIVATE study is assessing first-line therapy with 3 cycles of ibrutinib followed by 12 cycles of combined ibrutinib plus venetoclax in CLL/SLL patients aged ≤ 70 years. CAPTIVATE contains a fixed-duration cohort and an MRD cohort that was treated according to MRD-guided randomization after the completion of the 12 ibrutinib/venetoclax cycles (Figure 5). Overall, 149 individuals were enrolled into this cohort. Patients who achieved confirmed uMRD (n = 86; 58 %) were randomized to either ibrutinib or placebo and those with unconfirmed uMRD (n = 63; 42 %) to either ibrutinib plus venetoclax or ibrutinib monotherapy. Randomizations were double-blind for those with confirmed uMRD and open-label for the unconfirmed uMRD group.
Confirmed uMRD was defined as uMRD <10–4 by 8-color flow cytometry over at least 2 assessments at least 3 months apart and in both peripheral blood and bone marrow. Most patients showed high-risk features including del(17p)/TP53 mutation, complex karyotype, and unmutated IGHV. Ghia et al. reported the updated findings obtained in the MRD cohort at ASH 2021 [14]. At the time of the analysis, the median post-randomization follow-up was 24.0 months.
The treatment was shown to provide deep and durable responses. In the group that had already achieved uMRD after ibrutinib/venetoclax, none of the patients receiving either ibrutinib or placebo experienced new MRD relapses, disease progression or deaths in the additional year of follow-up after the primary analysis presented at ASH 2020 [15]. Both patients with and without uMRD obtained high PFS rates irrespective of their treatment; all four arms showed 3-year PFS rates of ≥ 95 %. At 36 months, 99 % of all patients were alive.
Figure 5: MRD cohort of the CAPTIVATE study: MRD-guided randomization
Potential for treatment-free remission
The greatest CR rate improvements noted in the MRD cohort of the CAPTIVATE trial occurred during the first year of randomized treatment. In patients with unconfirmed uMRD, increases in CR rates were similar with ibrutinib/venetoclax and ibrutinib alone. Likewise, the greatest uMRD rate improvements emerged during the first year of randomized therapy in this group, with ibrutinib/venetoclax inducing more pronounced benefits than single-agent ibrutinib. uMRD rates improved in a similar manner in patients achieving CR and partial response (PR).
Adverse events remained consistent with the known profiles for single-agent ibrutinib and venetoclax after the extended follow-up. Grade ≥ 3 AEs were infrequent across the randomized arms with the exception of neutropenia. The overall pre-randomization prevalence decreased across the four arms over time. Any-grade atrial fibrillation and major hemorrhage occurred in 10 % and 2 %, respectively. Dose reductions or discontinuations after randomization were uncommon.
According to the authors’ conclusion, the results obtained in patients with confirmed uMRD support the potential for treatment-free remission with first-line, fixed-duration ibrutinib/venetoclax treatment. Moreover, early data suggest that patients who progress after fixed-duration ibrutinib/venetoclax can be successfully retreated with single-agent ibrutinib. These findings were obtained in the MRD placebo arm and the fixed-duration cohort; here, all 9 patients with available responses achieved PR.
GLOW study: ibrutinib/venetoclax
Munir et al. presented the MRD outcomes and correlation with PFS observed in the phase III GLOW study that tested ibrutinib/venetoclax for 12 cycles after a 3-cycle ibrutinib lead-in compared to 6 cycles of chlorambucil plus obinutuzumab in elderly or unfit patients [16]. CLL patients without del(17p) or known TP53 mutation participated in this study; they were either ≥ 65 years old or < 65 years with CIRS scores > 6 or creatinine clearance < 70 mL/min. The primary analysis after a median follow-up of 27.7 months had yielded a 78 % reduction in the risk of progression or death (median PFS, not reached vs. 21 months; HR, 0.216; p < 0.0001) [17]. For the current analysis, MRD was evaluated with next generation sequencing and reported with cutoffs of < 10-4 and < 10-5.
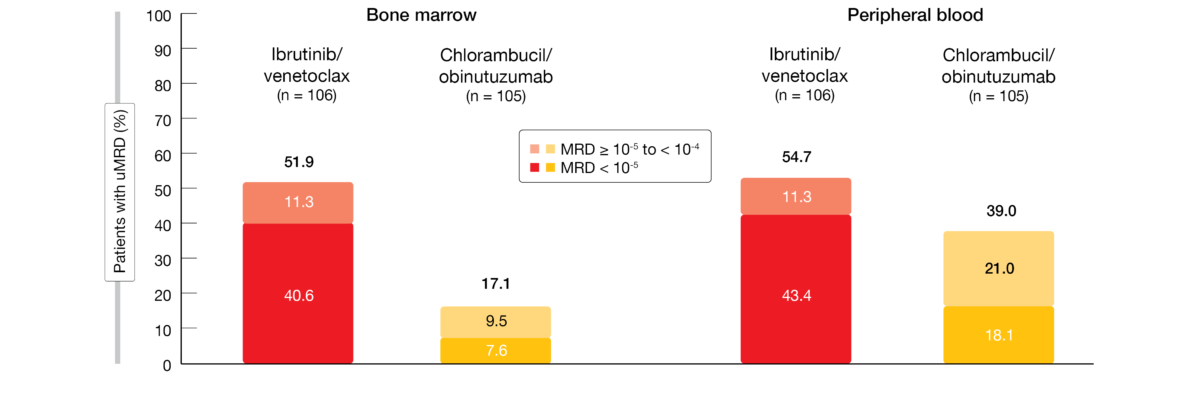
After 34.1 months of follow-up, superior PFS was maintained with ibrutinib/venetoclax vs. chlorambucil/obinutuzumab (HR, 0.212; p < 0.0001). At 30 months, the PFS rates amounted to 80.5 % vs. 35.8 %. Three months after the end of treatment, the uMRD < 10-4 rates were significantly higher with ibrutinib/venetoclax than with chlorambucil/obinutuzumab in both bone marrow (51.9 % vs. 17.1 %; p < 0.0001) and peripheral blood (54.7 % vs. 39.0 %; p = 0.0259). In the experimental arm, but not in the control arm, most patients with MRD < 10-4 had deep responses of < 10-5 (Figure 6). Depth of MRD response was similarly pronounced in peripheral blood and bone marrow for patients with unmutated IGHV; also, among patients with mutated TP53, 5 of 7 achieved uMRD < 10-5 in both blood and marrow with ibrutinib/venetoclax. Assessment of the MRD dynamics after the end of treatment showed that molecular and clinical relapses were less frequent during the first year in the experimental arm as uMRD was sustained more efficiently than in the control arm. Sustained uMRD 10-4 was observed in 84.5 % vs. 29.3 %, and sustained uMRD 10-5 occurred in 80.4 % vs. 26.3 %. Detectable MRD ≥ 10-4 was less likely to worsen or lead to progression with ibrutinib plus venetoclax.
Figure 6: GLOW trial: superior uMRD < 10-5 rates with ibrutinib/venetoclax vs. chlorambucil/obinutuzumab in both bone marrow and peripheral blood
PFS according to response
In terms of correlation with PFS, the analysis demonstrated a less pronounced impact of CR/CRi vs. PR on PFS in the experimental arm. While the 30-month PFS rates remained > 85 % for patients with CR/CRi or PR here, most patients in the control arm who had obtained PR progressed on treatment. In patients with uMRD < 10-4 in the marrow 3 months after the end of treatment, the PFS rate was sustained more effectively after ibrutinib/venetoclax than after chlorambucil/obinutuzumab. Similarly, those with detectable MRD ≥ 10-4 fared better with the ibrutinib-based regimen, as PFS rates > 90 % were sustained during the first post-treatment year independent of the MRD status, while early relapses frequently occurred with chlorambucil/obinutuzumab. Moreover, lymph node responses were better maintained in the experimental arm over time in patients with detectable minimal residual disease.
In their summary of the results obtained in the GLOW study, the authors noted that the unique relationship between MRD status and PFS might be explained by broader clearance of multiple disease compartments resulting from complementary mechanisms of ibrutinib and venetoclax. Additional follow-up is warranted to confirm the longer-term impact of the MRD status on PFS.
REFERENCES
- Eichhorst B et al., A randomized phase III study of venetoclax-based time-limited combination treatments vs. standard chemoimmunotherapy in frontline chronic lymphocytic leukemia of fit patients: first co-primary endpoint analysis of the international intergroup GAIA (CLL13) trial. ASH 2021, abstract 71
- Shanafelt TD et al., Ibrutinib and rituximab provides superior clinical outcome compared to FCR in younger patients with chronic lymphocytic leukemia (CLL): extended follow-up from the E1912 Trial. ASH 2019, abstract 33
- Davids MS et al., Ibrutinib plus fludarabine, cyclophosphamide, and rituximab as initial treatment for younger patients with chronic lymphocytic leukaemia: a single-arm, multicentre, phase 2 trial. Lancet Haematol 2019; 6(8): e419-e428
- Davids MS et al., Longer term follow-up of a multicenter, phase 2 study of ibrutinib plus FCR as initial therapy for younger patients with CLL. ASH 2021, abstract 640
- Hillmen P et al., Ibrutinib plus rituximab is superior to FCR in previously untreated CLL: results of the phase III NCRI FLAIR trial. ASH 2021, abstract 642
- Guo Y et al., Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem 2019; 62: 7923-7940
- Tam CS et al., Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019; 134(11): 851-859
- Tam CS et al., Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica 2021; 106(9): 2354-2363
- Brown JR et al., Efficacy and safety of zanubrutinib in patients with treatment-naïve chronic lymphocytic leukemia or small lymphocytic lymphoma with del(17p): follow-up results from Arm C of the SEQUOIA (BGB-3111-304) trial. ASH 2020, abstract 1306
- Tam CS et al., SEQUOIA: results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma. ASH 2021, abstract 396
- Tedeschi A et al., Zanubrutinib in combination with venetoclax for patients with treatment-naïve chronic lymphocytic leukemia or small lymphocytic lymphoma with del(17p): early results from Arm D of the SEQUOIA (BGB-3111-3049 trial. ASH 2021, abstract 67
- Woyach JA et al., Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 2018; 379(26): 2517-2528
- Woyach JA et al., Long term results of A041202 show continued advantage of ibrutinib-based regimens compared with bendamustine plus rituximab chemoimmunotherapy. ASH 2021, abstract 639
- Ghia P et al., First-line treatment with ibrutinib plus venetoclax for chronic lymphocytic leukemia: 2-year post-randomization disease-free survival results from the minimal residual disease cohort of the phase 2 CAPTIVATE study. ASH 2021, abstract 68
- Wierda WG et al., Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma: 1-year disease-free survival results from the MRD Cohort of the phase 2 CAPTIVATE study. ASH 2020, abstract 123
- Munir T et al., First prospective data on minimal residual disease outcomes after fixed-duration ibrutinib plus venetoclax versus chlorambucil plus obinutuzumab for first-line treatment of CLL in elderly or unfit patients: the GLOW study. ASH 2021, abstract 70
- Kater A et al., Fixed duration ibrutinib and venetoclax versus chlorambucil plus obinutuzumab for first-line chronic lymphocytic leukemia: Primary analysis of the phase 3 GLOW study. EHA 2021, abstract LB1902
© 2022 Springer-Verlag GmbH, Impressum