Mantle cell lymphoma: refining clinical outcomes beyond the current boundaries
Parsaclisib: primary analysis of CITADEL-205
Targeted therapies including BTK inhibitors are used in the second- and later-line treatment of patients with mantle cell lymphoma (MCL), although intolerance and treatment failure are common, with poor survival outcomes in the relapsed and refractory setting [1, 2]. This highlights the need for novel agents such as the potent and highly selective next-generation PI3Kδ inhibitor parsaclisib. The phase II CITADEL-205 study assessed parsaclisib in patients with relapsed/refractory MCL previously treated with or without the BTK inhibitor ibrutinib. At ASH 2021, Mehta et al. presented the primary efficacy and safety analysis for the cohort of BTK-inhibitor–naïve patients [3].
These patients were originally divided into a weekly dosing group and a daily dosing group. After an interim analysis, enrollment was closed in the weekly dosing group. The recommended dose is parsaclisib 20 mg/d for 8 weeks followed by 2.5 mg/d. Data were reported for the daily dosing group (n = 77) and for all treated patients (n = 108) who included those that switched from 20 mg once weekly to 2.5 mg/d. The objective response rate (ORR) constituted the primary endpoint.
Tumor reductions in almost all patients
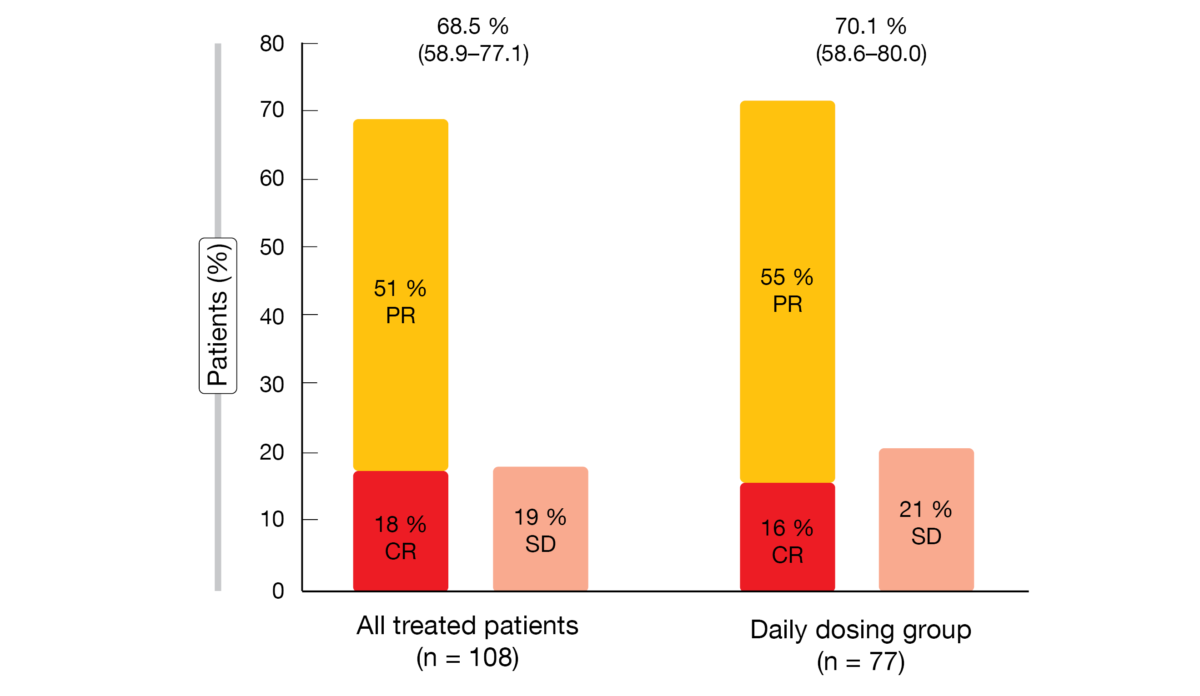
Parsaclisib showed excellent activity, with ORRs of approximately 70 % in both evaluated groups (Figure 1). In 89 % of all responders, responses were observed already at the time of the first disease assessment at 8 weeks. Regression of target lesions occurred in 96 % of evaluable patients, with 84 % showing > 50 % reductions in best percentage change from baseline. Progression-free survival was 13.6 and 12.0 months for the daily dosing group and all treated patients, respectively. Regarding duration of response, this was 12.1 and 13.7 months, respectively. Median overall survival had not been reached yet in either group. Eighty percent of patients were alive at 1 year.
In general, the patients tolerated the treatment well. Diarrhea was the most frequently observed adverse event (AE; 40 % and 34 % in the daily dosing group and all treated patients, respectively), followed by pyrexia (17 % and 18 %, respectively). The most common treatment-emergent AEs leading to dose interruption in the daily dosing group were diarrhea (14 %) and neutropenia (9 %). Dose discontinuation was mainly due to diarrhea (16 %) and colitis (6.5 %). Grade ≥ 3 diarrhea occurred in 18 % and 14 %, respectively, after a median of 5.1 and 4.3 months, respectively. For colitis, grade ≥ 3 events were seen in 5 % and 4 %, respectively, after a median of 3.1 months. Improvement to grade ≤ 2 was noted after approximately 11 days for diarrhea and 20 days for colitis. According to the authors, parsaclisib represents a potentially new treatment option for BTK-inhibitor–naïve patients with relapsed/refractory MCL and is a first-in-class PI3Kδ inhibitor in the setting of MCL.
Figure 1: Objective responses observed with parsaclisib in all treated patients and in the daily dosing group. PR, partial response; CR, complete response; SD, stable disease
TP53-mutant disease: BOVen
Patients with TP53-mutant MCL constitute a high-risk subset that shows poor survival when treated with intensive chemoimmunotherapy [4]. Given the lack of a standard frontline regimen, the combination of zanubrutinib, obinutuzumab, and venetoclax (BOVen) was hypothesized to be well tolerated and efficacious in this setting. It has already been demonstrated that the combination of a BTK inhibitor with a BCL2 inhibitor is synergistic and active in relapsed/refractory MCL, including patients with TP53 mutation [5]. Ibrutinib, obinutuzumab, and venetoclax has given rise to high response rates in relapsed and untreated MCL [6].
The preliminary analysis of a multicenter, investigator-initiated phase II study presented at ASH 2021 by Kumar et al. showed promising efficacy and good tolerability of BOVen in 17 patients with previously untreated MCL and TP53 mutation [7]. Fourteen patients were evaluable for efficacy. After a median follow-up of 4 months, the ORR was 86 %, with a CR rate of 64 %. At cycle 3, peripheral blood flow cytometry became negative in all patients. Grade ≥ 3 AEs occurred in 11 %. Serious AEs included grade 3 lung infection, grade 4 tumor lysis syndrome, and grade 1 non-neutropenic fever. No events resulted in drug discontinuation. Longer follow-up is required to further assess the outcomes over time.
Step-up dosing of glofitamab
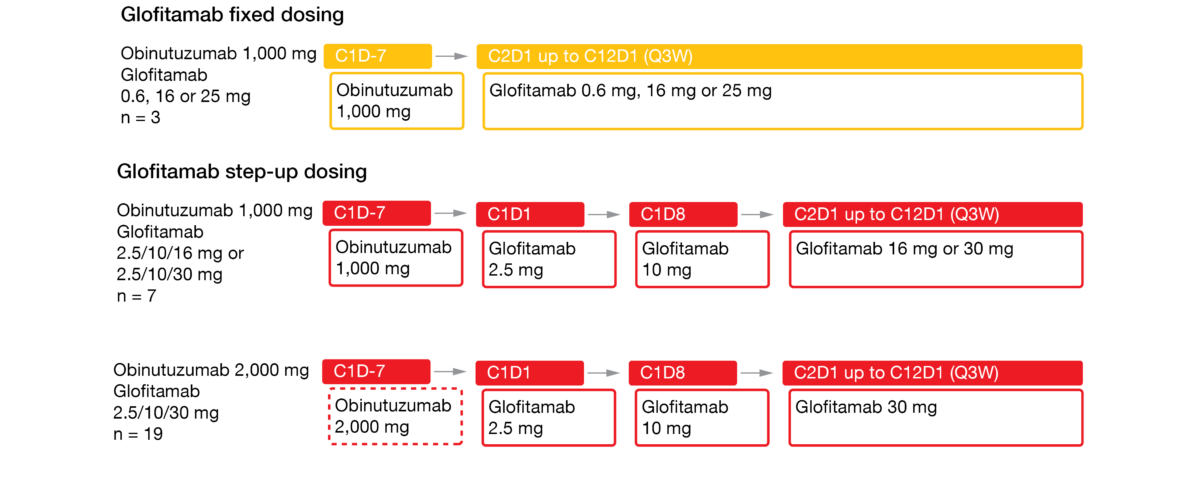
The bispecific antibody glofitamab redirects T cells to eliminate malignant B cells by targeting both CD20 and CD3 [8]. Glofitamab has shown promising efficacy and manageable safety as monotherapy and in combination with obinutuzumab in patients with heavily pretreated relapsed/refractory B-NHL [9, 10]. Obinutuzumab pretreatment and/or cycle 1 step-up dosing enabled effective mitigation of cytokine release syndrome (CRS). At ASH 2021, Phillips et al. reported data on obinutuzumab followed by glofitamab monotherapy in patients with relapsed/refractory MCL who were treated in the phase I dose escalation setting [11]. The study contained 3 arms. Glofitamab was assessed with fixed dosing after obinutuzumab 1,000 (n = 3), and with step-up dosing over 12 cycles (2.5 mg up to 30 mg) after obinutuzumab administered at doses of either 1,000 mg (n = 7) or 2,000 mg (n = 19) (Figure 2).
This approach gave rise to high response rates, with activity observed across the dosing regimens. For fixed dosing of glofitamab after obinutuzumab 1,000 mg, responses occurred in 67 %, and for step-up dosing after obinutuzumab 1,000 mg and 2,000 mg, this was 71 % and 91 %, respectively. Prior BTK inhibitor therapy did not affect the results; response rates in patients with and without prior BTK inhibition amounted to 83 % and 75 %, respectively. Overall, 81 % of patients in the entire group responded, and complete metabolic responses arose in 67 %. At the time of the data cutoff, most patients had ongoing responses, and 85.7 % of those with a CR remained in remission.
The treatment demonstrated favorable safety. Glofitamab-related grade 3/4 AEs occurred in 24.1 % of all patients. No fatal AEs or AEs leading to treatment discontinuation were reported. CRS represented the most common all-grade AE (58.6 %). Most CRS events were observed during cycle 1, were grade 1 or 2, and resolved. Immune effector cell-associated neurotoxicity syndrome (ICANS) grade 1 emerged in one patient (3.4 %) in the 1.000 mg obinutuzumab plus glofitamab step-up dosing cohort. No grade ≥ 2 ICANS or grade ≥ 3 tumor flare events were noted.
The authors pointed out that these results support a future confirmatory trial. Glofitamab, which is a fixed-duration regimen with off-the-shelf availability, continues to be evaluated in patients with relapsed/refractory MCL after BTK inhibitor treatment.
Figure 2: Design of the phase I dose escalation study assessing step-up dosing of glofitamab after obinutuzumab
Treatment patterns and health economics aspects
The retrospective US-based study conducted by Shah et al. examined characteristics of patients with MCL receiving BTK inhibitors, as well as treatment patterns and costs in the real-world setting [12]. Adult MCL patients with ≥ 1 BTK inhibitor prescription claim for 12 months were identified in the Symphony Health’s IDV, an open-source claims database. The final population consisted of 1,653 active patients on BTK inhibitor treatment. This analysis provides the first real-world evidence on patients with MCL treated with all currently available BTK inhibitors (i.e., ibrutinib, zanubrutinib, acalabrutinib).
The most common comorbidity at BTK inhibitor initiation was hypertension, followed by dyslipidemia and diabetes. NSAIDs and proton pump inhibitors were the two most common concomitant medications across the three drugs. More than half of the ibrutinib use took place in the frontline setting (68.4 %), while acalabrutinib and zanubrutinib were mainly used in relapsed/refractory disease (68.9 % and 80.6 %, respectively). Among zanubrutinib users, 22 % were switched from ibrutinib and 7 % from acalabrutinib; among acalabrutinib users, 21 % were switched from ibrutinib. The compliance rate was higher for zanubrutinib (65 %) than for acalabrutinib (62 %) and ibrutinib (59 %).
Among patients with ≥ 1 hospitalization, the average length of stay was found to be 5.9 days. Patients in the zanubrutinib group had shorter length of stay (4.0 days) than those treated with acalabrutinib (5.8 days) and ibrutinib (5.9 days). On average, the total submitted inpatient charge per stay was lower for patients in the zanubrutinib group (51,051 $) than for those in the acalabrutinib (74,546 $) and ibrutinib (79,482 $) groups. Future studies are needed to further understand factors associated with treatment selection and outcomes.
REFERENCES
- Epperla N et al., Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma-a “real world” study. Hematol Oncol 2017; 35(4): 526-535
- Jain P et al., Four-year follow-up of a single arm, phase II clinical trial of ibrutinib with rituximab (IR) in patients with relapsed/refractory mantle cell lymphoma (MCL). Br J Haematol 2018; 182(3): 404-411
- Mehta A et al., Efficacy and safety of parsaclisib in patients with relapsed or refractory mantle cell lymphoma not previously treated with a BTK inhibitor: primary analysis from a phase 2 study (CITADEL-205). ASH 2021, abstract 382
- Eskelund CW et al., TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood 2017; 130(17): 1903-1910
- Tam CS et al., Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med 2018; 378(13): 1211-1223
- Le Gouill S et al., Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood 2021; 137(7): 877-887
- Kumar A et al., Preliminary safety and efficacy from a multicenter, investigator-initiated phase II study in TP53-mutant mantle cell lymphoma with zanubrutinib, obinutuzumab, and venetoclax (BOVen). ASH 2021, abstract 3540
- Bacac M et al., CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res 2018; 24(19): 4785-4797
- Hutchings M et al., Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol 2021; 39(18): 1959-1970
- Morschhauser F et al., Glofitamab as monotherapy and in combination with obinutuzumab induces high complete response rates in patients with multiple relapsed or refractory follicular lymphoma. ASH 2019, abstract 128
- Phillips T et al., Glofitamab step-up dosing induces high response rates in patients with R/R MCL, most of whom had failed prior BTKi therapy. ASH 2021, abstract 130
- Shah B et al., Real-world Bruton tyrosine kinase inhibitor treatment patterns, compliance, costs, and hospitalizations in patients with mantle cell lymphoma in the United States. ASH 2021, abstract 3046
© 2022 Springer-Verlag GmbH, Impressum