Marginal zone lymphoma: PI3Kδ inhibition and beyond
CITADEL-204: parsaclisib in BTK-inhibitor–naïve patients
First-line treatment for patients with marginal zone lymphoma (MZL) typically includes anti-CD20-based regimens that generally evoke high response rates [1, 2]. However, in most cases, serial relapses eventually require several lines of therapy. The phase II CITADEL-204 trial evaluated the highly selective, next-generation PI3Kδ inhibitor parsaclisib in patients with relapsed/refractory MZL with or without prior exposure to ibrutinib. All patients had received ≥ 1 prior systemic therapy, including ≥ 1 anti-CD20 antibody.
Enrollment in the ibrutinib-experienced cohort was closed for feasibility reasons. Within the BTK-inhibitor–naïve cohort, patients were allocated into a weekly dosing group (i.e., parsaclisib 20 mg/d for 8 weeks followed by 20 mg once weekly) and a daily dosing group (i.e., parsaclisib 20 mg/d for 8 weeks followed by 2.5 mg/d continuously). Following an interim analysis, enrollment continued in the daily dosing group and was closed in the weekly dosing group. Daily dosing has been established as the recommended regimen. At ASH 2021, Phillips et al. reported the primary efficacy and safety analysis for all treated patients (n = 100) and the daily dosing group (n = 72) [3]. The entire treated cohort included patients who had switched from 20 mg once weekly to 2.5 mg/d.
Rapid and durable responses
Objective responses by independent review committee, which were defined as the primary endpoint, occurred in 58.0 % and 58.3 % of patients in the all-treated and daily dosing groups, respectively. Complete remissions were noted in 6 % and 4 %, respectively. Two thirds of all responders showed their first response already at the first disease assessment after 8 weeks. Comparable responses rates were observed in patients with nodal, extranodal, and splenic MZL. Median duration of response and median progression-free survival were 12.2 months and 16.5 months, respectively, for both the entire treated cohort and the daily dosing group. All evaluable patients had regression at target lesions or spleen, with 83 % experiencing > 50 % reduction in best percentage change from baseline.
Parsaclisib showed a manageable safety profile. Treatment-emergent adverse events (TEAEs) primarily included diarrhea (47 % and 53 % in all treated patients and the daily dosing group, respectively), cough (23 % and 26 %, respectively), and rash (18 % for both groups). Two deaths due to AEs (i.e., febrile neutropenia with sepsis/respiratory distress in one patient and sepsis in another) were deemed related to parsaclisib. The TEAEs most commonly leading to dose interruption were diarrhea (15 %) and neutropenia (6 %). Treatment discontinuation was mainly due to diarrhea (12.5 %) and colitis (7 %). Grade ≥ 3 diarrhea emerged in 12 % and 15 % in all treated patients and the daily dosing group, respectively, after a median of 5.6 and 5.1 months, respectively. Grade ≥ 3 colitis was reported in 7 % and 10 %, respectively, after a median of 5.6 months. Improvement to grade ≤ 2 occurred after 11-12 days for diarrhea and 21 days for colitis.
Favorable findings for U2
In relapsed/refractory MZL patients, the PI3Kδ inhibitor umbralisib as monotherapy has been shown to give rise to a 49.3 % objective response rate (ORR) with complete remissions in 16 % [4]. Umbralisib combined with the anti-CD20 antibody ublituximab (U2) has demonstrated clinical benefits in patients with relapsed/refractory non-Hodgkin lymphoma, with a manageable safety profile [5]. Chavez et al. presented results for the MZL cohort included in the global phase II UNITY-NHL study at ASH 2021 [6]. Patients with relapsed/refractory MZL who had been pretreated with ≥ 1 anti-CD20 agent received continuous umbralisib in combination with fixed-duration ublituximab for a maximum of 24 cycles. ORR by independent review committee constituted the primary endpoint. The efficacy-evaluable group contained 71 individuals.
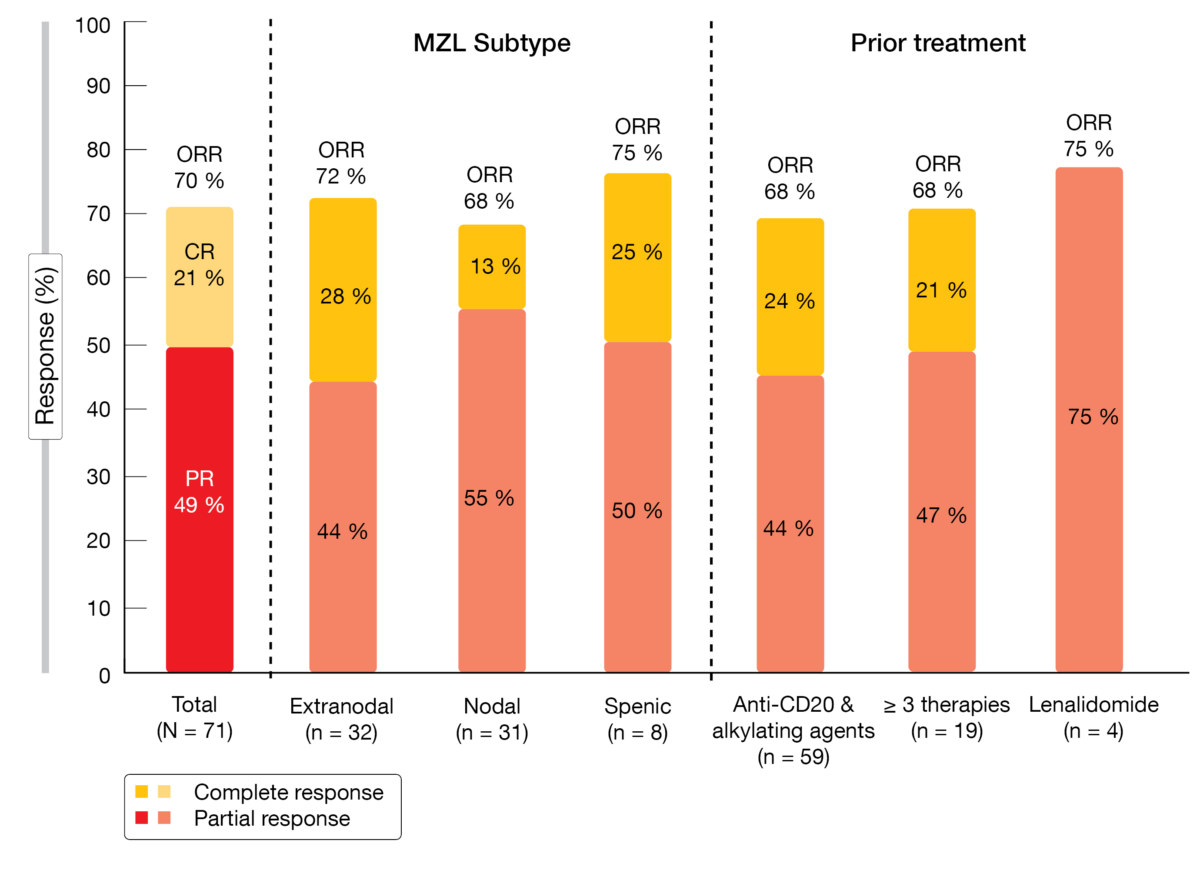
Overall, 70 % of these patients responded to treatment, with 21 % achieving complete remission (Figure). Disease control was obtained in 93 %. Responses were similar regardless of MZL subtype and prior treatment, as depicted in the Figure. At the time of the analysis, median duration of response had not been reached yet. Eighty-eight percent of patients experienced reductions in tumor burden from baseline. Median progression-free survival was 17.61 months; after censoring of COVID19-related deaths, this endpoint had not been reached yet.
The U2 regimen demonstrated an acceptable safety profile. Diarrhea occurred most commonly (49 %), followed by nausea (42 %) and fatigue (38 %). Dose reductions were resorted to as a measure for controlling AEs in 22 patients (31 %). Among PI3K-specific events, transaminase elevations and diarrhea were the main reasons for dose reductions (11 % and 4 %, respectively). Only 1 patient out of 9 who developed grade 3/4 diarrhea required steroids. Two cases of grade 3/4 non-infectious colitis occurred (2.8 %). As the authors pointed out, use of the U2 regimen resulted in increased response rates compared to the cohort previously treated with umbralisib alone. In all, the combination showed favorable clinical activity and might constitute a novel non-chemotherapy approach for patients with relapsed/refractory MZL.
Figure: Responses to umbralisib plus ublituximab: overall, according to MZL subtype and according to prior treatment
REFERENCES
- Denlinger NM et al., Management of relapsed/refractory marginal zone lymphoma: focus on ibrutinib. Cancer Manag Res 2018; 10: 615-624
- Lumish M et al., How we treat mature B-cell neoplasms (indolent B-cell lymphomas). J Hematol Oncol 2021; 14: 5
- Phillips T et al., Efficacy and safety of parsaclisib in patients with relapsed or refractory marginal zone lymphoma: primary analysis from a phase 2 study (CITADEL-204). ASH 2021, abstract 44
- Zinzani PL et al., Umbralisib, the once daily dual inhibitor of PI3Kδ and casein kinase-1ε demonstrates clinical activity in patients with relapsed or refractory indolent non-Hodgkin lymphoma: results from the phase 2 global Unity-NHL trial. ASH 202, abstract 2934
- Lunning M et al., Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2019; 134(21): 1811-1820
- Chavez JC et al., The combination of umbralisib plus ublituximab is active in patients with relapsed or refractory marginal zone lymphoma: results from the phase 2 global UNITY-NHL trial. ASH 2021, abstract 45
© 2022 Springer-Verlag GmbH, Impressum