Phase II data on novel BTK inhibitors for patients with Waldenström’s macroglobulinemia
Orelabrutinib: rapid and lasting responses
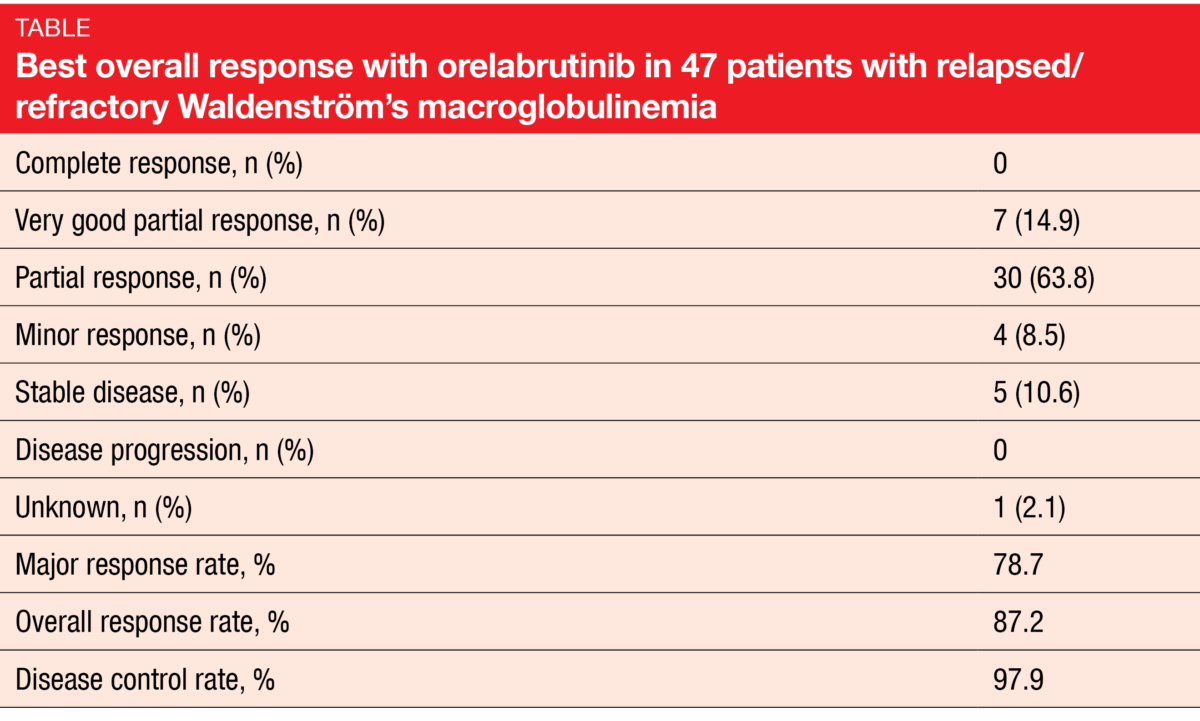
Second-generation BTK inhibitors such as orelabrutinib and tirabrutinib are currently being evaluated in the treatment of Waldenström’s macroglobulinemia (WM). Orelabrutinib is a BTK inhibitor with excellent target selectivity and almost 100 % BTK occupancy [1]. At ASH 2021, Zhou et al. reported the results for 47 patients with relapsed/refractory WM who received orelabrutinib 150 mg/d in the single-arm, multicenter, open-label, phase II ICP-CL-00105 study [2]. The major response rate (MRR; complete, partial, and very good partial responses) was defined as the primary endpoint.
Major responses were achieved quickly, after a median of 1.99 months. The MRR was 78.7 %, and the overall response rate amounted to 87.2 % (Table). Disease control resulted in 97.9 %. Remissions proved durable, which was mirrored by the 12-month rates: for major responses, this was 91.3 %, and for responses in general, 92.6 %. Subgroup analyses showed a consistent treatment effect across the prespecified groups. At 12 months, 93.6 % of participants were alive, and 89.3 % were progression-free. Durable improvement in hemoglobin level was found in 78.7 %, with a median maximal increase of 33 g/L. For serum IgM levels, the median reduction from baseline amounted to 79.7 %.
Orelabrutinib demonstrated a favorable safety profile, with relatively low rates of off-target toxicities. The most common adverse events (AEs) included thrombocytopenia (all grades, 27.7 %), hemorrhage (27.7 %), infections (21.3 %), and neutropenia (19.1 %), which were mostly mild to moderate. No treatment-emergent grade ≥ 3 events were reported for diarrhea, atrial fibrillation/flutter, hypertension, and hemorrhage. Treatment-related AEs prompted dose reduction and study drug discontinuation in 6.4 % and 2.1 %, respectively. Summarizing these findings, the authors noted that orelabrutinib has the potential to be the agent of choice for patients with relapsed/refractory WM.
Two-year update for tirabrutinib
Tirabrutinib, a BTK inhibitor with kinase selectivity comparable to or greater than other BTK inhibitors, has already been approved in Japan for use in treatment-naïve or relapsed/refractory WM based on the results of a phase II study [3, 4]. Cohort A of this trial included 18 treatment-naïve patients, while Cohort B consisted of 9 patients with relapsed/refractory WM. Tirabrutinib was administered orally under fasting conditions at a daily dose of 480 mg. The MRR constituted the primary endpoint. According to the primary analysis, the trial met the primary endpoint despite the relatively short follow-up [4]. In Cohort A, MRR and overall response rate were 88.9 % and 94.4 %, respectively. In Cohort B, these amounted to 88.9 % and 100 %, respectively.
The updated results after a 2-year follow-up presented at ASH 2021 showed that responses deepened over time [5]. At data cutoff, 83 % and 78 % of patients in Cohorts A and B, respectively, were still on treatment. All patients were alive at 24 months, and freedom from progression was present in 94.4 % and 88.9 %, respectively. The MRR was 94.4 % and 88.9 %, respectively. Overall, 94.4 % and 100 % of patients responded. Median duration of response had not been reached yet in either cohort. Patients who remained on treatment demonstrated continued reductions in tumor size and serum IgM levels.
The most common AEs of special interest were skin-related disorders. Rash occurred in 44.4 % in the total population (61.1 % and 11.1 % in Cohorts A and B, respectively) but was restricted to grade 1 and 2. In 57 % of cases, the patients developed skin-related AEs within the first month of treatment; no onset was observed after 7 months. Neutropenia ranged second among the AEs, with 16.7 % and 66.7 % in treatment-naïve and pretreated patients, respectively. During the extended follow-up period, no new grade ≥ 3 treatment-related AEs were noted except for hypertriglyceridemia (3.7 %). The authors concluded that tirabrutinib is a useful treatment option for patients with Waldenström’s macrogobulinemia.
REFERENCES
- https://cn.innocarepharma.com/en/media/press-release/20211203/
- Zhou D et al., Efficacy and safety of orelabrutinib in relapsed/refractory Waldenström’s macroglobulinemia patients: a multicenter, open-label, phase II study. ASH 2021, abstract 46
- Kaptein A et al., Potency and selectivity of BTK inhibitors in clinical development for B-cell malignancies. Blood 2018; 132 (Suppl 1): 1871
- Sekiguchi N et al., A multicenter, open-label, phase II study of tirabrutinib (ONO/GS-4059) in patients with Waldenström’s macroglobulinemia. Cancer Sci 2020; 111(9): 3327-3337
- Suzuki K et al., Two-year follow-up data of phase II study of tirabrutinib, a second-generation Bruton’s tyrosine kinase inhibitor, in patients with treatment-naïve or relapsed/refractory Waldenström’s macroglobulinemia. ASH 2021, abstract 1352
© 2022 Springer-Verlag GmbH, Impressum