Real-world risk assessment, outcomes and adoption of novel drugs in CLL patients: insights from US databases
Testing patterns after diagnosis
Prognostic testing including IGHV mutation status, cytogenetic abnormalities by FISH, and immunophenotyping has been recommended after diagnosis of CLL/SLL prior to treatment initiation. This also applies to previously treated patients in some settings. As disease with high-risk genetic features is better managed with novel agents than with chemoimmunotherapy, the need for testing has recently become more relevant as all patients are advised to complete risk-factor testing for both prognostication and selection of optimal, evidence-based therapy. Chanan-Khan et al. performed a retrospective evaluation of real-world patterns of testing which showed that despite these recommendations, a significant number of patients do not undergo FISH and/or IGHV mutation status testing prior to therapy [1]. Based on the Flatiron Health EHR-derived database, a total of 3,037 newly diagnosed patients with CLL/SLL were identified between July 2014 and February 2021. Their median age was 73 years, and 62.3 % were male. The majority (92 %) received treatment in community practices, with 54.1 % being commercially insured.
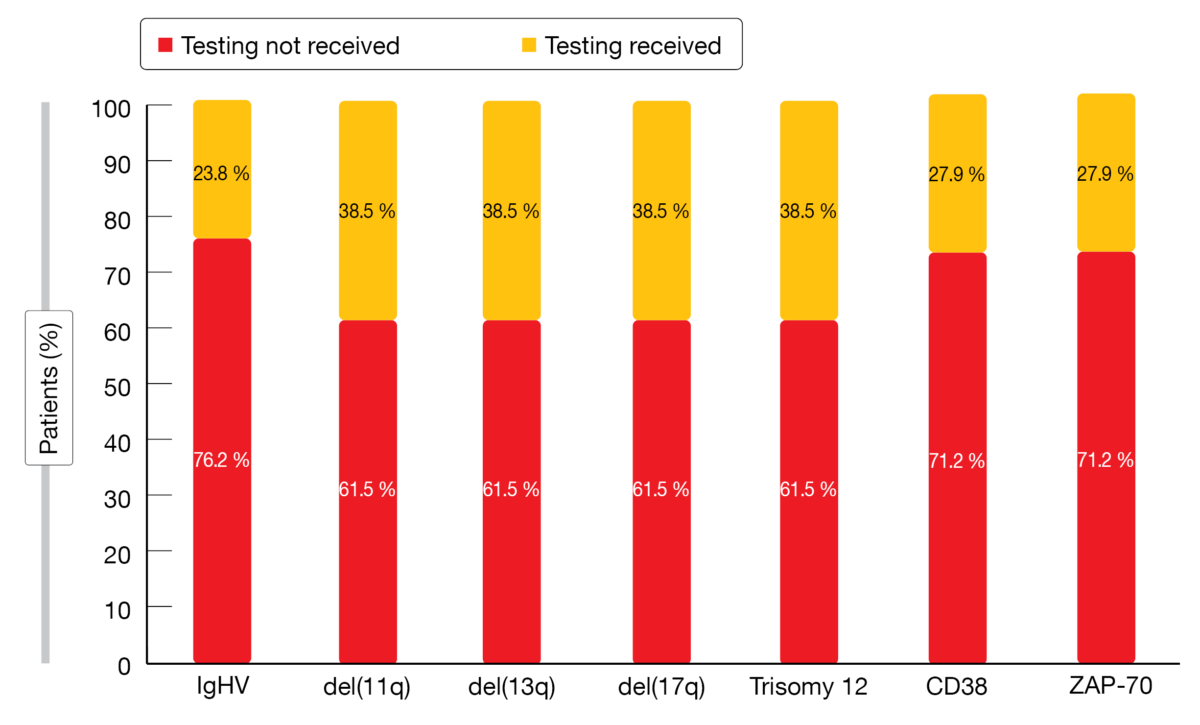
Over half of these patients did not receive risk factor testing (Figure 1). Almost all of those who underwent testing had it done once prior to the initiation of first-line therapy. Older individuals, females, and those living in the west of the US were significantly less likely to receive IGHV and FISH testing. A multivariable analysis revealed that patients who lived in the northeast or west had decreased likelihood to receive immunophenotyping tests.
Figure 1: Real-world frequency of risk factor testing in patients with newly diagnosed CLL/SLL
Differences according to age and gender
Among those who underwent testing, the presence of high-risk biomarkers was as follows: unmutated IGHV, 56.1 %; del(17p), 14.4 %; del(11q), 16.9 %; CD38, 30.8 %. Compared to patients < 65 years of age, testing in patients ≥ 65 years demonstrated a lower presence of unmutated IGHV and del(11q), while del(17p) and trisomy 12 were identified more frequently. No significant disparity was observed for white vs. non-white patients, except for a lower incidence of mutated IGHV and del(13q). Compared to tested men, tested women had lower rates of unmutated IGHV, del(11q), and CD38, while del(17p) was found more commonly.
The authors went on to investigate the impact of risk testing on therapy selection. According to this, patients with del(17p) had a higher likelihood of receiving novel agents including ibrutinib, acalabrutinib, and venetoclax than those who tested negative. In contrast, 26.4 % of those in whom del(17p) was identified and 39.8 % of those who did not get tested received chemotherapy. Overall, these data did not only identify a significant gap in testing but showed that suboptimal assessment is more common in vulnerable populations. The authors pointed out that there is an unmet need for further education and refinement of clinical practice, which is necessary to achieve the best clinical outcome through robust risk-assessment testing and optimal therapeutic triaging.
Impact of atrial fibrillation
Atrial fibrillation (AF) is a common type of arrhythmia and increases the risk of other cardiovascular complications such as stroke, bleeding events and heart failure. The clinical and economic impact of AF was assessed by Mohan et al. in CLL patients who are typically older and therefore likely to develop this type of arrhythmia [2]. Newly diagnosed CLL patients were identified in the IBM MarketScan Treatment Pathway from January 2009 to July 2020. Among 23,756 patients, 11.07 % had AF within 1 year of CLL diagnosis. Compared to CLL patients without AF, they were older on average (median age, 82 vs. 67 years), and more patients were male (65.1 % vs. 56.9 %).
Patients with AF demonstrated significantly increased prevalence of stroke (12.67 % vs. 4.97 %), bleeding events (17.45 % vs. 8.53 %), and heart failure (31.14 % vs. 4.7 %). They showed significantly higher median rates of emergency room visits (46 % vs. 24 %) and inpatient admissions (45 % vs. 19 %); overall, they were twice as likely to be hospitalized. Moreover, significant effects became apparent with respect to median outpatient, pharmacy, and total costs. The cost ratio for AF vs. no AF was 1.44 (p < 0.0001). According to the authors, better disease management, monitoring for AF, and improved CLL therapeutics with a lower AF risk or cardiovascular toxicity are required to minimize the incidence of AF in CLL.
Treatment in older patients
Given limited information on prescribing habits or characteristics of individuals receiving CLL treatment, Onukwugha et al. conducted an analysis using Medicare Claims data to characterize CLL treatment patterns and timing of treatment and to identify factors associated with the receipt of CLL treatment in patients aged ≥ 65 years [3]. The final sample included 3,440 individuals of whom 16 % (n = 556) were treated for CLL during follow-up (median, 540 days). Median time to receipt of treatment was 61 days.
Gender and age displayed a statistically significant association with treatment receipt. Males were more likely to be treated than females (55 % vs. 45 %; p < 0.01). Those aged 65-74 and 75-84 years received treatment more frequently than those ≥ 85 years (43 % and 42 % vs. 15 %, respectively), with a significant difference between the oldest and youngest groups (p < 0.01). The most common agents administered comprised ibrutinib (35 %) and rituximab (34 %) monotherapies. Less than half of the patients treated with bendamustine/rituximab completed six doses.
CLL/SLL-associated burden among veterans
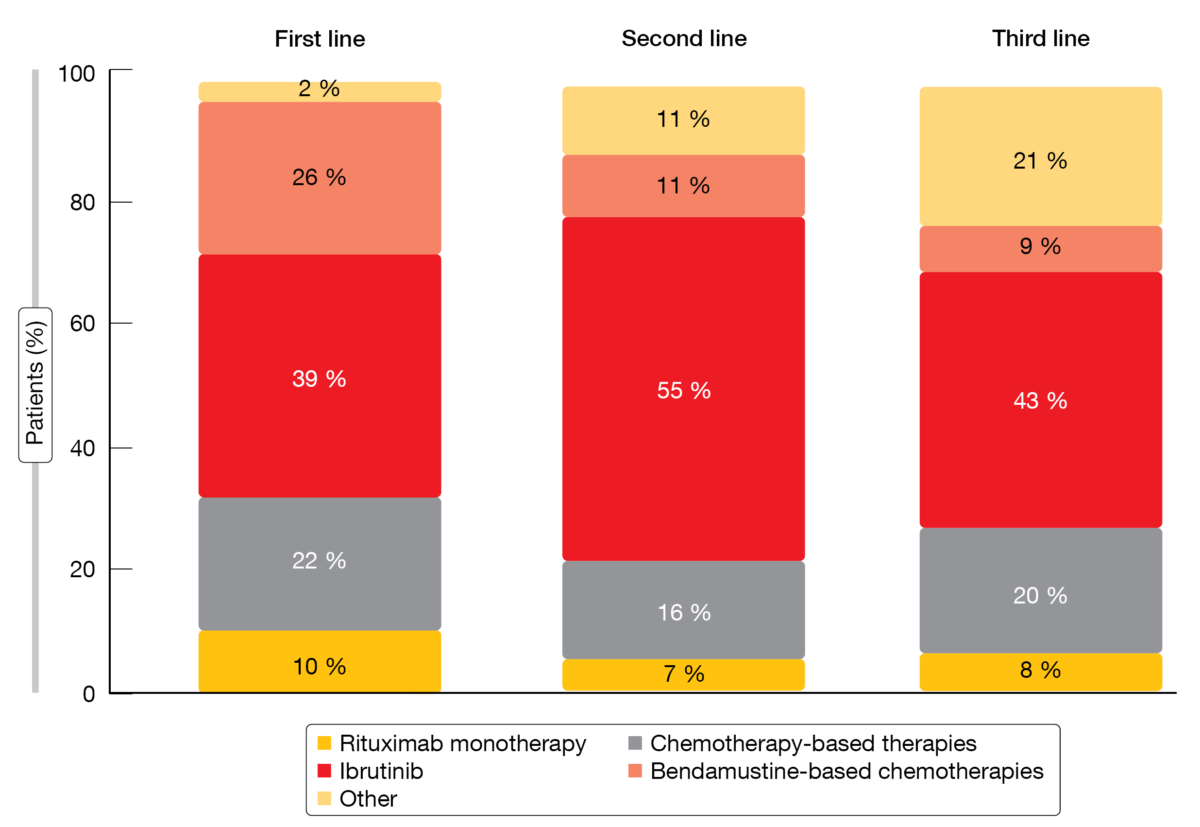
Using the Veteran Health Administration dataset, a retrospective analysis assessed the real-world CLL/SLL-related burden in US veterans, who are at increased risk of these diseases especially after exposure to Agent Orange or other herbicides during military service [4]. Also, the clinical and economic outcomes associated with current treatments were evaluated. Between October 2014 and September 2019, 13,664 veterans newly diagnosed with CLL/SLL were identified. The final study population consisted of 2,861 patients with a median age of 70 years who received ≥ 1 line of CLL/SLL therapy. Average time to first-line treatment initiation from diagnosis was 315 days. In 26.9 %, the patients went on to receive second-line therapy, and third-line treatment was administered in 7.0 %. Ibrutinib was the most common treatment regimen across all lines (Figure 2).
Overall, treatment discontinuation rates were high across current regimens in each treatment line (73 %, 66 % and 59 %, respectively). They were generally highest for bendamustine-based chemotherapies, with 85 %, 84 % and 89 % of patients discontinuing in the first, second and third lines, respectively. The overall treatment switching rate was highest in third line (26 %), followed by 23 % in second line and 10 % in first line. Hospitalizations due to CLL/SLL occurred in 39 %, with an average length of stay of 7 days. Total all-cause and CLL/SLL-related per-patient-per-month healthcare costs increased by treatment line. After adjustment for patient clinical and demographic covariates, the analysis showed that treatment discontinuation and switches were statistically significant predictors of more frequent inpatient admissions and increased length of hospital stay.
In their summary, the authors noted that these real-world data demonstrated significant clinical and economic burden associated with CLL/SLL among the US veterans. The suboptimal adherence, as reported by the high treatment discontinuation rates, and its impact on costs and healthcare resource use, highlights the real-world unmet needs of CLL/SLL management in the veteran population.
Figure 2: Treatment patterns among patients with CLL/SLL by line of therapy
REFERENCES
- Chanan-Khan A et al., Real-world patterns for risk assessment and implications on the adoption of novel therapeutics in chronic lymphocytic leukemia: IGHV mutation status, FISH cytogenetics, and immunophenotyping. ASH 2021, abstract 4078
- Mohan A et al., Impact of atrial fibrillation on cardiovascular and economic outcomes in patients with chronic lymphocytic leukemia. ASH 2021, abstract 4077
- Onukwugha E et al., Factors associated with treatment among older adults diagnosed with chronic lymphocytic leukemia: an analysis using Medicare Claims data. ASH 2021, abstract 1968
- Yang K et al., Real-world treatment patterns, adherence and healthcare resource utilization for chronic lymphocytic leukemia/small lymphocytic lymphoma among veterans in the United States. ASH 2021, abstract 4079
© 2022 Springer-Verlag GmbH, Impressum