Active monotherapies and combinations in mantle cell lymphoma
First-line triple combination: ALR
The initial treatment of patients with mantle-cell lymphoma (MCL) is continuously evolving due to the introduction of new targeted agents. Ruan et al. conducted a single-arm phase II study based on the hypothesis that the addition of the next-generation BTK inhibitor acalabrutinib to lenalidomide plus rituximab (ALR) would synergize activity and accelerate minimal residual disease (MRD)-negative complete response (CR), thus allowing for response-adapted adjustment of treatment intensity to minimize toxicity.
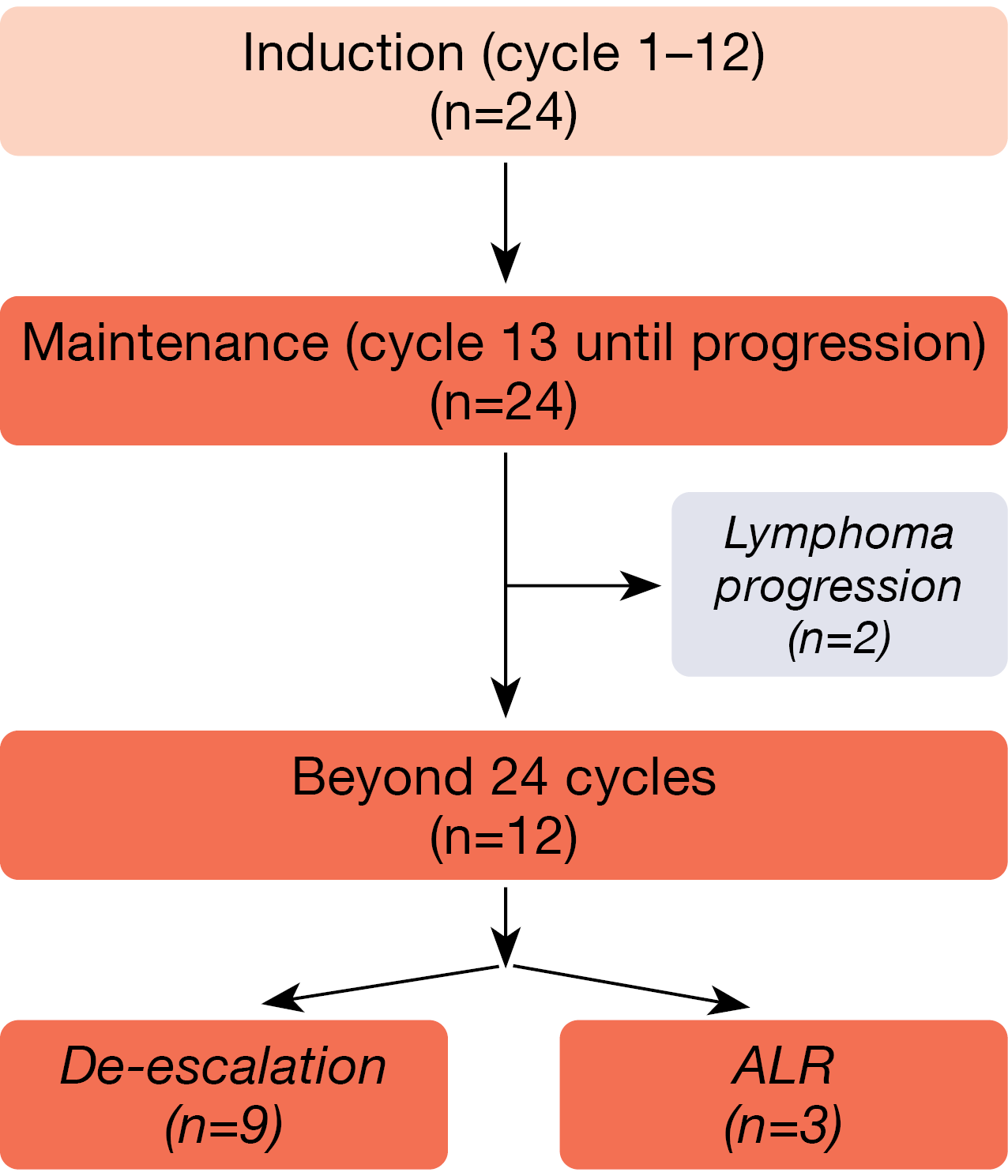
The study included patients with untreated MCL and a tumor mass diameter of ≥ 1.5 cm. Those who benefited from induction (cycles 1–12) moved on to maintenance (cycle 13 until disease progression). Patients who achieved MRD-negative CR during maintenance had the option of treatment de-escalation after 24 cycles, i.e., discontinuation of the oral agents acalabrutinib and lenalidomide. Retreatment was possible in case of progression. Patients in whom MRD positivity persisted continued the triple combination. CR at the end of induction was defined as the primary endpoint.
At ASH 2022, data were presented after a median follow-up of 23 months for 24 patients with stage III/IV MCL who received ALR including maintenance [1]. Twelve of these had completed more than 24 cycles of treatment at the time of the analysis (Figure 1).
Figure 1: Acalabrutinib plus lenalidomide/rituximab: patient flow in the phase II setting.
High CR and molecular response rates
The overall response rate (ORR) estimated at the end of induction was 100 %, while CR was achieved in 83 %. Molecular remission was measured using MRD clono–SEQ®, with the detection limit set at < 1 x 10-6. According to this, most patients obtained MRD-negative CR. At the conclusion of induction, the MRD negativity rate was 67 %; this increased to 83 % at cycle 24. De-escalation was possible in 9 of 12 patients. The 2-year rates for progression-free survival (PFS) and overall survival (OS) were 86.7 % and 100 %, respectively.
Using next-generation sequencing, the researchers assessed the genomic mutation profile. The most common mutations included IGLL5, ATM, CCND1 and TP53 mutations. Five of six patients with TP53 mutations achieved MRD negativity within 12 cycles, although one of them relapsed at cycle 20. MIPI score, Ki-67 index, or TP53 mutational status did not affect response, while OS appeared to correlate with the TP53 mutational status. These exploratory data suggest that TP53 mutations might be associated with risk of lymphoma progression following initial response.
The treatment was well tolerated, with expected side effects that mainly included asymptomatic neutropenia, anemia, and thrombocytopenia. No cases of febrile neutropenia occurred. Most patients had COVID-19 infections, although all of them recovered with supportive care. Other adverse events (AEs) comprised rash, fatigue, and gastrointestinal side effects. The authors concluded that real-time MRD analysis facilitates response-adapted treatment de-escalation during maintenance to minimize toxicity, which warrants further evaluation in larger studies. An expansion cohort receiving acalabrutinib plus lenalidomide and obinutuzumab is currently being launched (NCT03863184).
AVR: phase I results
Acalabrutinib in addition to venetoclax and rituximab (AVR) as first-line treatment in patients with MCL was assessed in the multicenter, open-label, phase IB ACE-LY-106 study that has already yielded high rates of clinical and molecular responses [2]. Wang et al. presented an update for 21 patients after a median follow-up of 25.8 months at ASH 2022 [3].
AVR was shown to be well tolerated and safe. The most common AEs were diarrhea, headache and fatigue, which were mostly grade 1 and 2. Grade 3/4 AEs included neutropenia (33 %) and pneumonia (14 %). Serious any-grade AEs occurred in 57 %; these were COVID-19 infection (24 %), pneumonia (14 %), and pyrexia (10 %). Four of seven patients contracting COVID-19 died from the infection. Temporary dose holds and permanent discontinuation of a study drug due to AEs were noted in 71 % and 24 %, respectively.
All of the patients responded to the treatment. The CR rate with bone marrow confirmation (Lugano criteria) was 71 %, while the CR rate according to PET/CT amounted to 90 %. Median duration of response had not been reached yet, which also applied to median PFS and OS. At 2 years, 74 % of patients were alive, and 54 % were progression-free.
MRD data from peripheral blood were available for 16 patients. Fourteen of these (87.4 %) achieved MRD negativity at least once during treatment. At cycles 6 and 12, 57.1 % and 66.7 % were MRD-negative, respectively. Among the 15 patients who obtained CR by PET/CT, 11 (73.3 %) remained in CR at their last follow-up. As the authors noted in their summary, these findings warrant further investigations of AVR in the treatment-naïve setting.
Glofitamab monotherapy in heavily pretreated patients
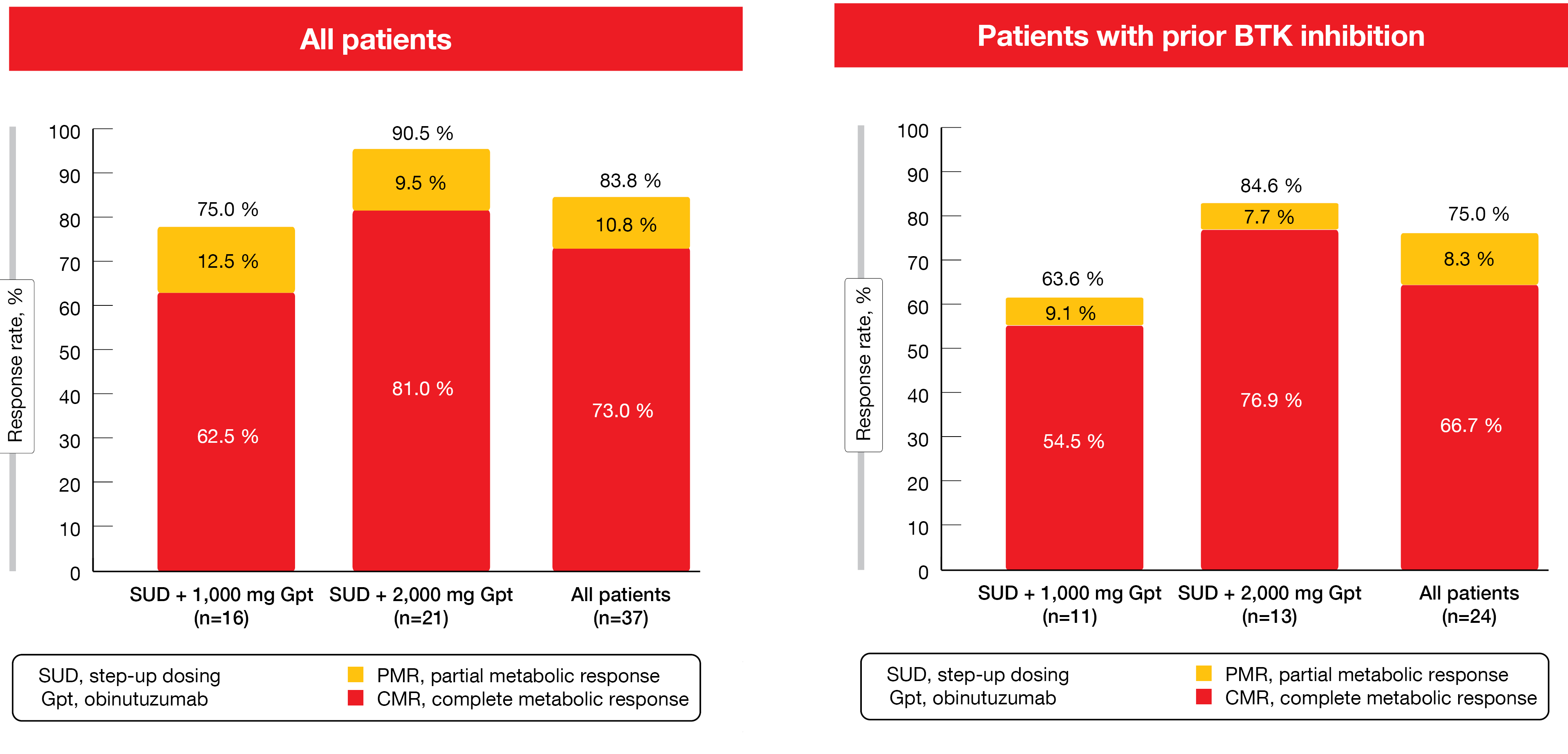
In the setting of relapsed/refractory MCL, patient prognosis is poor, particularly after BTK inhibition [4]. Data from a phase I dose-escalation study presented by Phillips et al. demonstrated benefits of the CD20/CD3 bispecific antibody glofitamab in heavily pretreated patients with relapsed/refractory MCL [5]. Glofitamab was tested as mono–therapy for a maximum of 12 cycles at a dose of 30 mg in cycles 2–12 after step-up dosing in cycle 1 (2.5 mg and 10 mg on days 8 and 15). For reasons of cytokine release syndrome (CRS) mitigation, the administration of obinutuzumab 1 x 1,000 mg or 1 x 2,000 mg preceded the treatment. While 16 patients received glofitamab after obinutuzumab 1,000 mg, 21 were treated with glofitamab after obinutuzumab 2,000 mg. The majority had previously received BTK inhibitor therapy and was refractory to any prior treatment.
Glofitamab monotherapy gave rise to high response rates in the overall population and in patients with prior BTK inhibitor therapy (Figure 2). Most responses were achieved early on and were durable. Median time to first response was 51 days. CRs lasted for a median of 10.0 months. At data cut-off, 74.1 % of patients with CR remained in remission. After the exclusion of four COVID-19-related deaths, median duration of CR had not been reached, and 87 % of CRs were ongoing. Moreover, durable CRs were maintained after cessation of therapy.
Glofitamab-related grade 3/4 AEs emerged in 62.2 %. No grade 5 AEs related to glofitamab or AEs leading to treatment discontinuation occurred. The 2,000 mg obinutuzumab dose, as compared to the 1,000 mg dose, was associated with a lower rate of serious glofitamab-related AEs (52.4 % vs. 75.0 %) and grade 3/4 AEs (52.4 % vs. 87.5 %). CRS was the most common AE but was manageable and predominantly classified as grade 1 or 2. The cohort treated with obinutuzumab 2,000 mg experienced fewer instances of CRS than the cohort receiving obinutuzu–mab 1,000 mg (66.7 % vs. 87.5 %), and no grade-4 events were reported in the higher dose group. Other AEs of interest included infections, neutropenia and tumor flare. No grade ≥ 3 immune effector cell-associated neurotoxicity syndrome (ICANS) or tumor flare events occurred. A phase III study investigating glofitamab monotherapy is currently being planned for patients with relapsed/refractory MCL.
Figure 2: Complete and partial metabolic responses with glofitamab monotherapy in the total cohort and in the BTK-inhibitor–pretreated patients
Second- vs. later-line zanubrutinib
Pooled data of 112 patients with relapsed/refractory MCL from the phase I BGB-3111-206 study and the phase II BGB-3111-AU-003 study that evaluated the next-generation BTK inhibitor zanubrutinib in the second line (n = 41) and in later lines (n = 71) have shown numerically improved PFS and OS with second-line zanubrutinib compared to later-line treatment [6]. The long-term follow-up analysis conducted at 35.2 months further confirmed these findings [7].
Inverse propensity score weighting was used to balance the baseline covariates. After weighting, OS was significantly improved in the second-line group vs. the later-line group (HR, 0.459; p = 0.044). Median OS had not been reached yet in either arm, and the 36-month OS rates were 82.0 % vs. 66.5 %. Second- and later-line groups experienced similar reductions in the risk of progression or death (HR, 0.78), although median PFS in the second-line cohort was numerically longer (27.8 vs. 22.1 months). At 36 months, 44.8 % vs. 35.4 % of patients were progression-free. The OS and PFS findings in the original sample were consistent with the post-weighting results. Both groups responded to a similar extent (ORRs, 88.6 % vs. 85.7 %), with median duration of response amounting to 25.2 vs. 25.1 months.
Furthermore, the safety profile was similar (all-grade AEs, 95.1 % vs. 97.2 %). Grade ≥ 3 AEs of special interest including atrial fibrillation/flutter, diarrhea, hemorrhage and hypertension occurred only infrequently in either group. Taken together, the data suggested that earlier treatment with zanubrutinib is associated with improved survival outcomes.
REFERENCES
- Ruan J et al., Phase 2 trial of acalabrutinib-lenalidomide-rituximab (ALR) with real-time monitoring of MRD in patients with treatment-naïve mantle cell lymphoma. ASH 2022, abstract 73
- Wang M et al., Safety and efficacy of acalabrutinib plus venetoclax and rituximab in patients with treatment-naïve mantle cell lymphoma. Blood 2021; 138(Supplement 1): 2416
- Wang M et al., Acalabrutinib plus venetoclax and rituximab in patients with treatment-naïve mantle cell lymphoma: 2-year safety and efficacy analysis. ASH 2022, abstract 2884
- Martin P et al., Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016; 127(12): 1559-1563
- Phillips T et al., Glofitamab monotherapy induces high complete response rates in patients with heavily pretreated relapsed or refractory mantle cell lymphoma. ASH 2022, abstract 74
- Zhou K et al., Zanubrutinib monotherapy in relapsed/refractory mantle cell lymphoma: a pooled analysis of two clinical trials. J Hematol Oncol 2021; 14(1): 167
- Song Y et al., Zanubrutinib treatment in patients with relapsed/refractory MCL: an updated pooled analysis. ASH 2022, abstract 2894
© 2023 Springer-Verlag GmbH, Impressum
More posts
Potential strategies to overcome relapsed and refractory Waldenström’s macroglobulinemia
Potential strategies to overcome relapsed and refractory Waldenström’s macroglobulinemia
Preface – ASH 2022
Preface – ASH 2022 © Lebens.Med Zentrum Bad Erlach - Stefan Vogt, MD, Lebens.Med Zent