Follicular lymphoma: bispecific and PI3Kδ-targeted approaches
Advanced-stage follicular lymphoma (FL) remains incurable, with most patients eventually experiencing disease progression despite therapeutic advances. Relapsed or refractory FL is challenging to treat, particularly in high-risk patients who are refractory to prior treatments and have progressed within 24 months. The combination of rituximab and lenalidomide (R2) is commonly used in this setting, although complete response (CR) rates are suboptimal [1]. This raises the need for novel, efficacious and well tolerated agents.
pcoritamab plus R2 in relapsed/refractory FL …
Epcoritamab, a subcutaneously administered CD3/CD20 T-cell–engaging, bispecific antibody is being investigated together with R2 in the phase I/II
EPCORE NHL-2 study based on the rationale that epcoritamab and R2 might synergize and have non-overlapping toxicities [2-4]. Arm 2b of the EPCORE NHL-2 study contains patients with relapsed/refractory CD20-positive, grade I–IIIA, stage II-IV FL. Epcoritamab 48 mg is administered weekly in cycles 1 and 2 followed by Q4W treatment for up to 2 years. At the same time, the patients receive rituximab until cycle 5 and lenalidomide for a maximum of 12 cycles. Among the 76 patients included in the analysis, 59 % had stage IV disease, and 51 % had FLIPI scores 3–5. Primary refractory and double refractory FL was present in 38 % and 39 %, respectively. In 42 %, progression had occurred within 24 months of the initiation of first-line treatment (POD24). Thirty-eight percent were refractory to their last line of therapy.
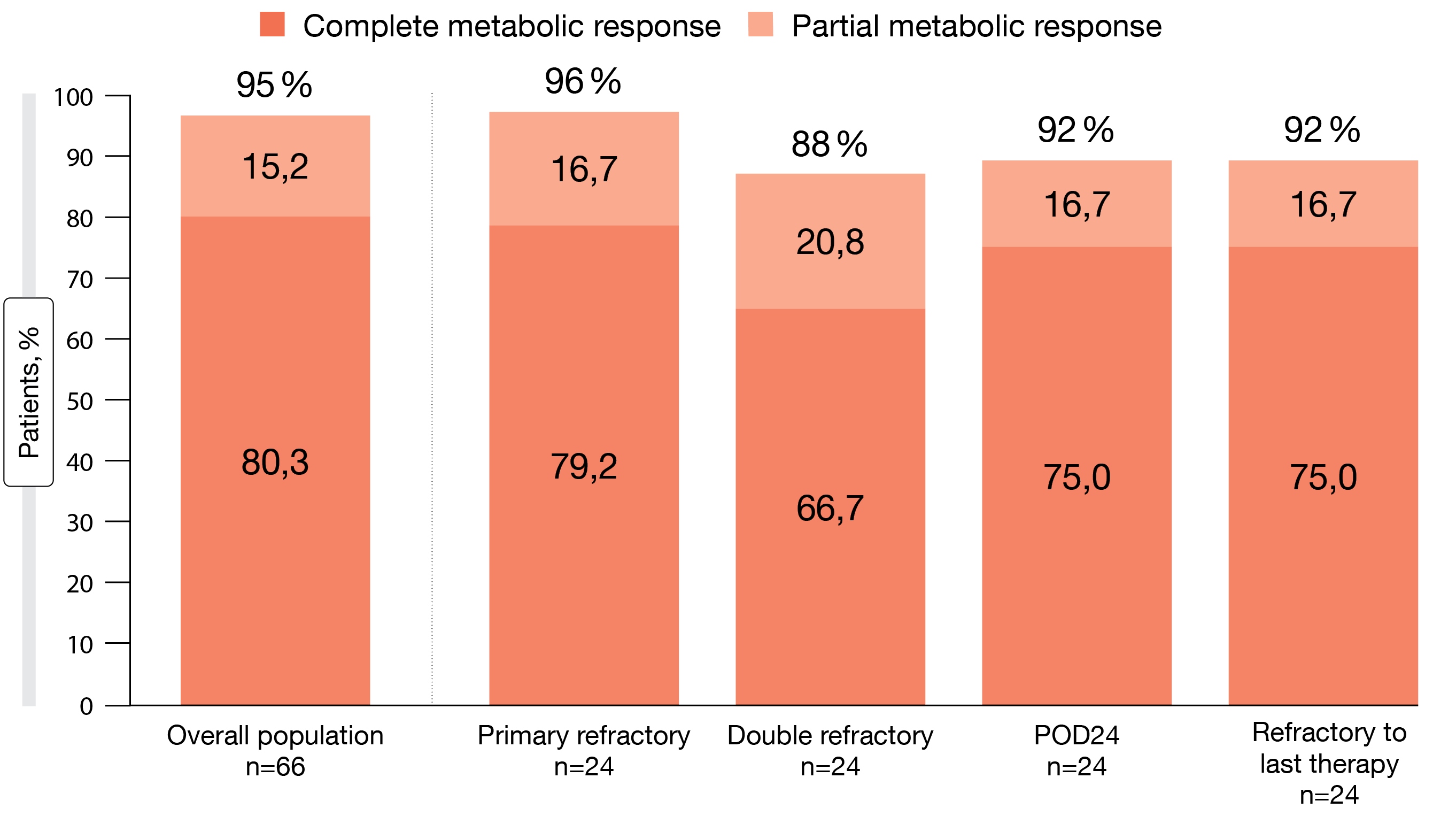
In this massively pretreated cohort, the combination of epcoritamab and R2 showed potent antitumor activity according to the update presented at ASH 2022 by Falchi et al. [5]. Overall, responses occurred in 96.2 %, and complete metabolic responses were seen in 83.5 %. Treatment was ongoing in 88 % of patients at the time of the analysis, and median duration of response had not been reached. Notably, the majority of remissions were obtained at the first assessment already, which made for a median time to response of 1.4 months. Moreover, deep responses occurred across high-risk subgroups including double refractory patients and those with a history of POD24 (Figure 1).
The safety of the combined treatment remained consistent with previous reports. Epcoritamab-related treatment-emergent adverse events (TEAEs) led to dose delays in 25 %, while none of these TEAEs gave rise to discontinuation. Neutropenia was the most common TEAE (all grades, 47 %); grade 3 and grade 4 neutropenic events were reported in 20 % each. Cytokine release syndrome (CRS) occurred in 43 %, although none of these patients experienced grade ≥ 3 events, and CRS did not necessitate treatment discontinuation. Most CRS events emerged after the first full dose. The authors pointed out that these data support outpatient administration. Furthermore, no cases of clinical tumor lysis syndrome were observed. The ongoing phase III EPCORE FL-1 trial is evaluating fully outpatient epcoritamab plus R2 treatment in patients with relapsed/refractory FL (NCT05409066).
Figure 1: Responses to epicoritamab across high-risk subgroups with relapsed/refractory follicular lymphoma
… and treatment-naïve disease
Arm 6 of the EPCORE NHL-2 study is investigating epcoritamab + R2 in adults with previously untreated CD20-positive, grade 1 I–IIIA FL. In the expansion phase, 41 patients received epcoritamab weekly in cycles 1 and 2 and Q4W from cycle 3 for up to 2 years combined with rituximab weekly in cycle 1 followed by Q4W administration in cycles 2–6 and oral lenalidomide in cycles 1–12. Almost all patients had stage III/IV disease, and FLIPI scores 3–5 were present in one third of the population.
As the first analysis of the data from arm 6 of the EPCORE NHL-2 study showed, epcoritamab + R2 gave rise to high overall response and complete metabolic response rates of 94 % and 86 %, respectively [6]. Almost all responses had occurred at the time of the first assessment and were maintained, with 80 % of patients still being on treatment after a median follow-up of 8.1 months. Median duration of response had not been reached yet.
No new safety findings were reported. CRS showed predictable timing, with most events noted after the first full dose, and was restricted to grade 1 and 2. Dose delays and treatment discontinuation related to epicoritamab therapy were seen in 17 % and 7 %, respectively. No clinical tumor lysis syndrome emerged. In their summary, the authors emphasized that these results support further clinical evaluation of epcoritamab + R2 as a chemotherapy-free regimen in previously untreated patients with FL.
Single-agent mosunetuzumab
The CD20/CD3 bispecific antibody mosunetuzumab is being assessed in a pivotal single-arm, multicenter, phase II study that includes patients with relapsed or refractory grade I–IIIA FL who had received ≥ 2 systemic therapies including an anti-CD20 antibody and an alkylator. Mosunetuzumab was administered as fixed-duration treatment at a dose of 30 mg Q3W after step-up dosing in cycle 1. In patients who achieved CR after 8 cycles, treatment was discontinued; in case of partial response or stable disease, mosunetuzumab was administered for a maximum of 17 cycles. As this treatment can be performed in the outpatient setting, hospitalization was not mandatory. Ninety patients participated most of whom had stage III/IV disease. After a median of 3 lines of prior therapy, 69 % were refractory to their last treatment, and 79 % were refractory to any prior anti-CD20 therapy. In 52 %, a history of POD24 was present. Mosunetuzumab therapy gave rise to an overall response rate (ORR) of 80 % and a complete response (CR) rate of 60 %, which meant that the primary endpoint of CR superiority vs. historical control was met [7].
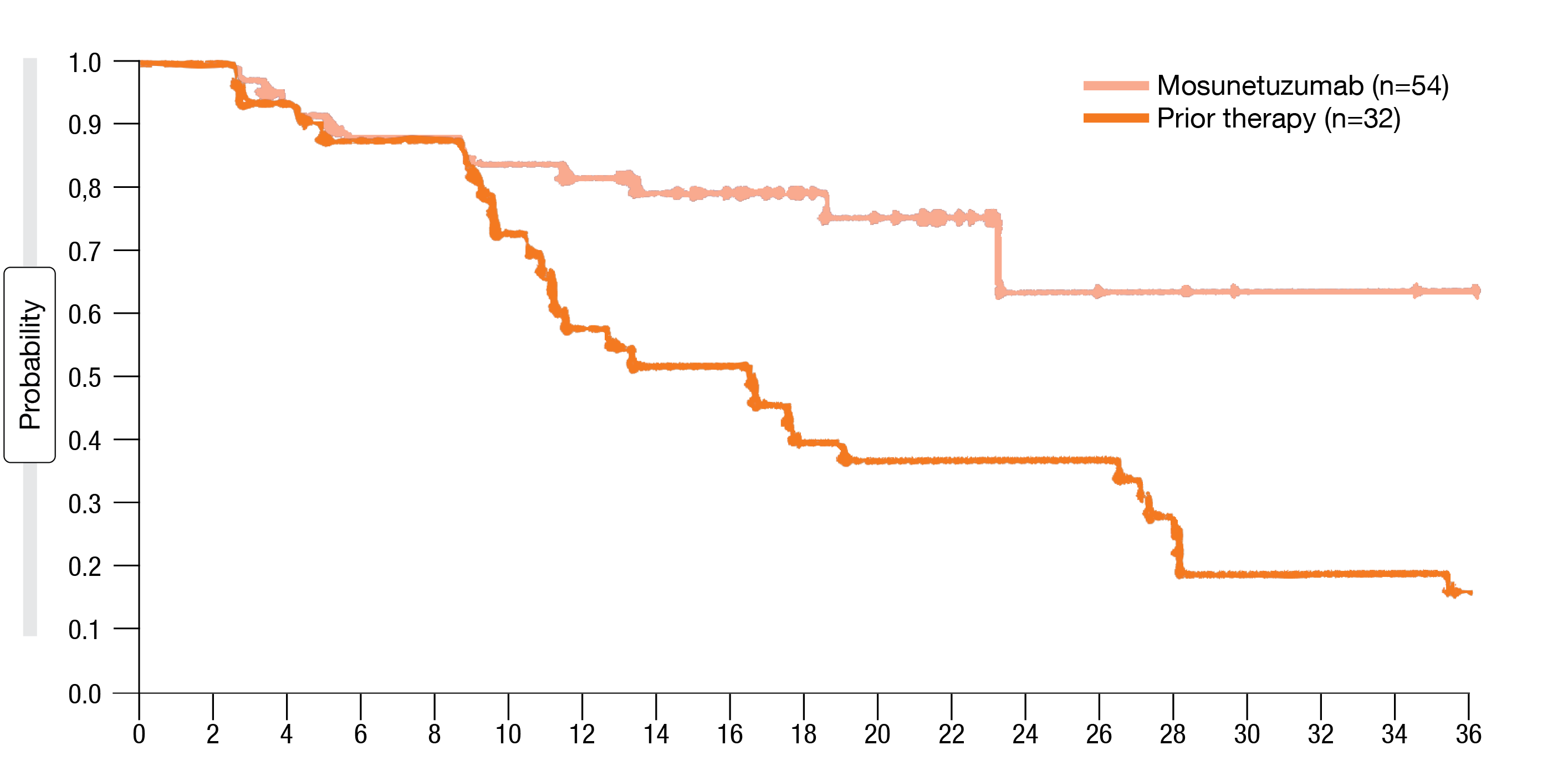
The updated outcomes presented at ASH 2022 after a median follow-up of 28.3 months demonstrated that only 18 % of patients required treatment beyond 8 cycles [8]. Overall, 78 % responded and 60 % obtained CR, which was consistent with the published findings [7]. Responses proved durable; at 24 months, 63 % of patients were in ongoing CR. Median overall survival and median time to next treatment had not been reached yet. Clinically meaningful response rates were observed in patients with common mutations including those associated with poor prognosis, such as TP53 mutations. Furthermore, mosunetuzumab induced substantially improved response rates compared to the last prior therapy (ORR, 78 % vs. 56 %; CR, 60 % vs. 36 %), with longer duration of CR (median, not reached vs. 15 months; Figure 2) and improved progression-free survival (24 vs. 12 months).
The analysis demonstrated a manageable safety profile without new serious AEs, grade ≥ 3 AEs, or treatment-related AEs. No additional CRS events had been noted. Occurrence of CRS and tumor response showed no correlation. In their conclusion, the authors emphasized that mosunetuzumab is a promising option as an off-the-shelf, outpatient therapy with fixed duration of treatment.
Figure 2: Duration of complete remission with mosunetuzumab vs. prior treatment
Intermittent dosing of zandelisib
The clinical pharmacology of the oral PI3Kδ inhibitor zandelisib allows for dosing on a non-continuous schedule [9]. Zelenetz et al. reported results for patients with FL grade I-IIIA who received zandelisib 60 mg on an intermittent schedule in the open-label, phase II TIDAL study. Zandelisib was administered daily during cycles 1 and 2 (i. e., the induction phase) followed by dosing on days 1–7 of 28-day cycles from cycle 3 onward. The patients showed progressive disease after ≥ 2 prior therapies, which must have included an anti-CD20 antibody and an alkylating agent. Overall, 121 individuals were analyzed. This was a heavily pretreated population, with a median of 3 previous therapies. Almost all had received prior chemoimmunotherapy, and 56.2 % had a history of POD24.
Single-agent zandelisib gave rise to high rates of durable remissions. Overall, 72.7 % of patients responded, and CR resulted in 38.0 %. Eighty-five percent achieved disease control. Median duration of response and median progression-free survival were 16.4 and 11.6 months, respectively. Remissions occurred in patients with poor prognostic factors such as history of POD24 (ORR, 73.5 %) and refractory disease (ORR, 68.5 %). In the group after 2 prior lines of treatment, the ORR was 84.2 %, with CR achieved in 43.9 %. Moreover, remissions were seen early, as 87.5 % of responses emerged by the end of cycle 2. Almost all patients with post-baseline disease assessment experienced tumor reduction.
Grade ≥ 3 TEAEs of special interest primarily occurred in cycles 1–3, which represented the continuous dosing period and the washout phase during the first intermittent dosing cycle, while their cumulative incidence decreased substantially thereafter. Diarrhea represented the most common TEAE, followed by nausea, fatigue, pyrexia and neutropenia. In 13.2 %, patients discontinued treatment due to treatment-related AEs.
As the authors pointed out, these data support the evaluation of zandelisib on an intermittent dosing schedule as a single agent or in combination in various B-cell malignancies, both in relapsed disease and earlier lines of treatment. The phase III COASTAL trial investigating zandelisib plus rituximab vs. chemoimmunotherapy is ongoing in patients with relapsed/refractory FL and marginal zone lymphoma (NCT04745832).
REFERENCES
- Leonard JP et al., AUGMENT: A phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol 2019; 37(14): 1188-1199
- Engelberts PJ et al., DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine 2020; 52: 102625
- Chiu CW et al., Preclinical evaluation of epcoritamab combined with standard of care therapies for the treatment B-cell lymphomas. Cancer Res 2021; 81(13_Supplement): 1574-1574
- Falchi L et al., Subcutaneous epcoritamab with rituximab + lenalidomide (R2) in patients with relapsed or refractory follicular lymphoma: Update from phase 1/2 trial. J Clin Oncol 40, 2022 (suppl 16; abstr 7524)
- Falchi L et al., Subcutaneous epcoritamab with rituximab + lenalidomide in patients with relapsed or refractory follicular lymphoma: phase 1/2 trial update. ASH 2022, abstract 609
- Falchi L et al., Subcutaneous epcoritamab in combination with rituximab + lenalidomide (R2) for first-line treatment of follicular lymphoma: initial results from phase 1/2 trial. ASH 2022, abstract 611
- Budde LE et al., Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol 2022; 23(8): 1055-1065
- Bartlett NL et al., Mosunetuzumab monotherapy demonstrates durable efficacy with a manageable safety profile in patients with relapsed/refractory follicular lymphoma who received ≥ 2 prior therapies: updated results from a pivotal phase II study. ASH 2022, abstract 610
- Moreno O et al., Safety, pharmacokinetics, and pharmacodynamics of ME-401, an oral, potent, and selective inhibitor of phosphatidylinositol 3-kinase P110δ, following single ascending dose administration to healthy volunteers. Clin Ther 2018; 40(11): 1855-1867
- Zelenetz AD et al., TIDAL: phase 2 study of the efficacy and safety of single-agent zandelisib administered by intermittent dosing: final result of the relapsed or refractory follicular lymphoma cohort. ASH 2022, 1563
© 2023 Springer-Verlag GmbH, Impressum
More posts
Clinical findings with sundry targets in various B-cell malignancies
Clinical findings with sundry targets in various B-cell malignancies AUGMENT: 5-year r
Follicular lymphoma: bispecific and PI3Kδ-targeted approaches
Follicular lymphoma: bispecific and PI3Kδ-targeted approaches Advanced-stage follicular
New approaches in relapsed and refractory DLBCL
New approaches in relapsed and refractory DLBCL Polatuzumab vedotin plus R-ICE Approxi
Chronic lymphocytic leukemia: moving towards new horizons
Chronic lymphocytic leukemia: moving towards new horizons Final analysis of ALPINE: za
Further steps to improve efficacy and safety in acute myeloid leukemia
Further steps to improve efficacy and safety in acute myeloid leukemia Long-term follo
Active monotherapies and combinations in mantle cell lymphoma
Active monotherapies and combinations in mantle cell lymphoma First-line triple combin