Quality of life as an endpoint
Quality of life (QoL) assessment adds useful information to the efficacy and safety data obtained in clinical studies. It contributes to the evaluation of different treatments and identifies patients who might benefit from supportive interventions. QoL data can be used to inform policy and resource allocation, reveal benefits to patients despite objective toxicity, and be of prognostic value. Evaluation of patient’s QoL might help to determine the suitable moment to start specific palliative interventions.
Patient numbers and patient compliance is an important factor; if they are not substantial at baseline, no reliable results can expected later on, as compliance tends to decrease over the course of the study. This is of particular significance with regard to long-term treatments like targeted therapies or immunotherapies.
Guidelines have introduced QoL as one factor to define the best treatment. Consequently, almost all of the clinical studies conducted today with the aim of evaluating a new treatment include one or two QoL assessments, and this has increased substantially over the last decades. QoL questionnaires are usually administered in conjunction with response evaluation. Outside of clinical trials, a single assessment can already be helpful.
Overall, the aspect of QoL is of great importance, as the impact of a malignant disease on a person’s life matters in the popular perception. This is reflected by a multitude of movies that deal with the topic of cancer.
Obstacles to QoL evaluation
However, in clinical practice, physicians frequently fail to assess QoL. They often feel that clinical judgement is sufficient and that testing takes too much time. Furthermore, they do not know which test to use and how to analyse and interpret it. An enormous range of QoL assessment instruments has cropped up over time. Also, a common perception is the assumption that the patients will get upset when being confronted with QoL assessments. Frequently it cannot be controlled whether the patient themselves or another person who has a different perception of the disease actually fills in the questionnaire. Trained nurses who hand out the questionnaires and explain about them to the patients might be helpful here. Translation can be another issue, as many questionnaires are being distributed to patients around the globe using “literal translation” without taking into account different cultures, attitudes and nuances of language.
On the level of clinical trials, the main challenge resulting from QoL evaluation is the drawing of conclusions, as several factors including the use of questionnaires, methods of data collection and data read-out differ between studies. Even if a substantial amount of data is available, these disparities impede the creation of generally valid statements.
Assessment tools
Overall, generic measurements (assessment of QoL with the same treatment in different diseases) are distinguished from specific ones (e.g., the Lung Cancer Symptom Scale [LCSS]). The decision which one to go for in a given case can be difficult. It appears sensible to use both generic and specific assessment tools, as this allows for a comprehensive evaluation of the patient.
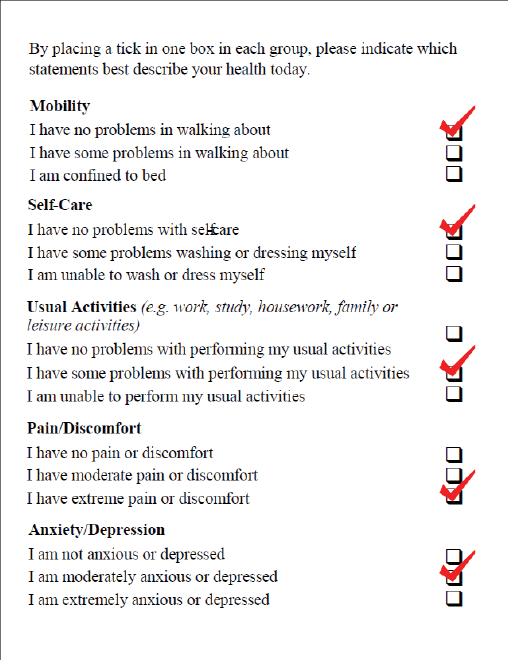
The EQ-5D is a generic measure of health status for patients with various diseases. It encompasses five dimensions (anxiety/ depression, mobility, pain/ discomfort, usual activities, self-care) (Figure 1). A patient’s overall health status is measured on a visual analog scale (VAS), which is easy to use. VAS use reduces the time of filling in questionnaires and can take place throughout the entire disease process until a patient’s death. Another example of a visual scale is the Edmonton Symptom Assessment System. It provides a rapid and simple assessment of symptom intensity, both in routine practice and in the context of research trials. Nine major symptoms (pain, nausea, tiredness etc.) have been defined.
The EORTC QLQ-C30 questionnaire was developed to assess QoL in cancer patients in general. It consists of five functional scales, three symptom scales, one global health status scale and six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties). Supplemental disease-specific modules are available for a range of distinct cancer types, as are many other generic instruments.
Figure 1: The EQ-5D Utility Index questionnaire
Lung-cancer-specific questionnaires
The EORTC QLQ-L13, which has been designed for lung cancer patients, incorporates one multi-item scale to assess dyspnea and a series of single items investigating cough, pain, sore mouth, dysphagia, peripheral neuropathy, alopecia, and use of pain medication. A recent update of the EORTC QLQ-L13 was established based on the results of an international, multi-center, phase III study [1]. The new module named EORTC QLQ-LC29 retains 12 of the 13 original LC13 items and includes new items that assess side effects of targeted therapy, radio-chemotherapy, and thoracic surgery.
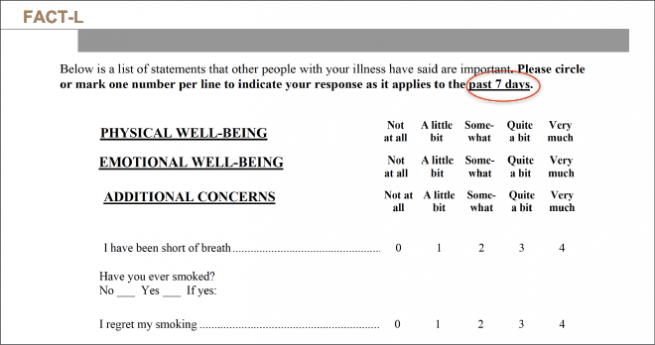
Likewise, FACT-L is specific for lung cancer. It comprises 36 questions and 5 domains and assesses physical, functional, emotional and social items, as well as pulmonary symptoms. Unlike other questionnaires, it contains a question as to whether the patient regrets smoking (Figure 4).
Figure 2: Design of the FACT-L questionnaire
Methods of administration
QoL evaluation can be performed in the setting of face-to-face interviews by trained interviewers, by telephone interviews, or by use of self-report questionnaires. Data collection is possible via both pencil and paper questionnaires and electronic instruments.
Naturally, elderly patients tend to have problems handling computers, but publications such as a study comparing the LCSS paper form with its electronic version (eLCSS-QL) revealed high acceptance [2]. Eighty-seven percent of patients required less than 3 minutes to learn how to use the electronic questionnaire. Good compliance was observed in the vast majority of cases, as well as reductions in the time of filling in the questionnaire. Also, the majority of the physicians and nurses involved in the study found all aspects of the eLCSS-QL easy to use and interpret. Application of the eLCSS-QL contributed to improvements with respect to the measurement of score ratings, collation of data for analysis, and maintenance of patient confidentiality thanks to the use of passwords. As no transcription of data is necessary, mistakes are avoided.
Patient-reported outcomes
Any outcome evaluated directly by the patient himself that is based on the patient perception of a disease and its treatment(s) is called patient-reported outcome (PRO) [3]. The term PRO has been proposed as an umbrella term to cover both single-dimension and multi-dimension measures relating to symptoms, health-related quality of life, health status, adherence to treatment, and satisfaction with treatment. PROs are of practical relevance because the patient and physician perceptions of various aspects such as the usefulness or toxicity of a certain treatment can deviate from each other to a considerable degree [4–6]. While physicians might rate a small OS advantage negligible, patients would accept chemotherapy if it substantially reduced symptoms even without prolonging life [4]. Expectations of therapy and AEs are important determinants for patient compliance [7].
In a tumor type such as lung cancer that is accompanied by debilitating physical and psychological symptoms, the study of symptom clusters is relevant to identify primary and secondary symptoms and to define their role for patient QoL. This approach can potentially improve the comprehensive QoL management in lung cancer patients. For instance, fatigue is particularly common and severe in lung cancer, reducing QoL in 57–100 % of patients from diagnosis to death [8]. The ASCO guidelines therefore recommend screening for fatigue in daily practice from the time of diagnosis, even if no questionnaires are routinely used [9].
REFERENCES
- Koller M, EORTC Update Lung Cancer Module Group. Update of the EORTC questionnaire for assessing quality of life in patients with lung cancer: Introducing the new EORTC QLQ-LC29. J Clin Oncol 2016; 34 (suppl; abstr e18096)
- Hollen PJ et al., Can a computerized format replace a paper form in PRO and HRQL evaluation? Psychometric testing of the computer-assisted LCSS instrument (eLCSS-QL). Support Care Cancer 2013; 21(1): 165-172
- European Medicines Agency, Reflection paper on the regulatory guidance for the use of health-related quality of life measures in the evaluation of medicinal products, 2005
- Silvestri G et al., Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. BMJ 1998; 317(7161): 771-775
- Novello S et al., Italian multicenter survey to evaluate the opinion of patients and their reference clinicians on the “tolerance” to targeted therapies already available for non-small cell lung cancer treatment in daily clinical practice. Transl Lung Cancer Res 2014; 3(3): 173-180
- Pacchiana MV et al., Patients’ attitudes and physicians’ perceptions toward maintenance therapy for advanced NSCLC: a multicenter Italian survey. WLCC 2015, ORAL27.02
- Cheung K et al., Reliability and validity of the Cancer Therapy Satisfaction Questionnaire in lung cancer. Qual Life Res 2016; 25(1): 71-80
- Gonzalez BD, Jacobsen PB. Depression in lung cancer patients: the role of perceived stigma. Psychooncology 2012; 21(3): 239-246
- Bower JE et al., Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 2014; 32(17): 1840-1850
Author: Silvia Novello
© 2017 Springer-Verlag GmbH, Impressum