EGFR-mutant lung cancer: what’s new with respect to activity and resistance?
Erlotinib as a neoadjuvant strategy
In patients with stage IIIA-N2 NSCLC, current multimodal treatment options include definitive chemoradiotherapy, surgery followed by adjuvant chemotherapy, or neoadjuvant treatment followed by surgical resection. The standard first-line EGFR tyrosine kinase inhibitor (TKI) erlotinib has already demonstrated feasibility in the neoadjuvant treatment setting of stage IIIA-N2 NSCLC [1]. Therefore, the open-label, randomised, phase II CTONG-1103 trial compared erlotinib with cisplatin-based chemotherapy as neoadjuvant/adjuvant treatment in patients with locally advanced, EGFR-mutant NSCLC [2]. The patients were randomised to either erlotinib 150 mg/d for 42 days (n = 37) or gemcitabine plus cisplatin three-weekly for 2 cycles (n = 35) prior to surgery. After the operation, patients in the experimental arm went on to receive erlotinib 150 mg/d for 12 months, while those in the control arm were treated with another 2 cycles of chemotherapy. ORR constituted the primary endpoint.
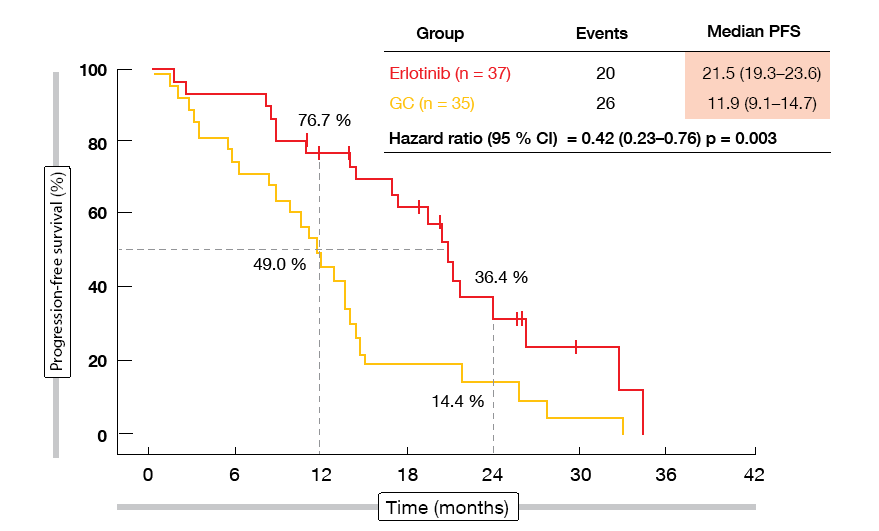
Neoadjuvant erlotinib indeed increased ORR, although not to a significant degree (54.1 % vs. 34.3 %; p = 0.092). The surgical outcomes favoured erlotinib numerically: a greater percentage of patients in the experimental group underwent surgery (83.8 % vs. 68.6 %; p = 0.129), and complete resections were more frequent in the erlotinib-treated arm (73.0 % vs. 62.9 %; p = 0.358), as was lymph node down-staging (10.8 % vs. 2.9 %; p = 0.185). Fifty surgically resected specimens were available. In both groups, no pathological complete responses occurred, but major pathological responses were obtained more often with erlotinib (10.7 % vs. 0 %). Erlotinib led to a significant improvement in PFS, which was defined as a secondary endpoint (21.5 vs. 11.9 months; HR, 0.42; p = 0.003; Figure 1). OS data had not yet reached maturity at the time of the analysis. Postoperative complications were balanced across the two groups, and adverse events associated with neoadjuvant/adjuvant therapy corresponded to those reported previously. As the authors concluded, the regimen warrants further exploration in the neoadjuvant setting.
Figure 1: Erlotinib versus chemotherapy in the neoadjuvant setting: progression-free survival advantage observed with the EGFR TKI therapy
First-line gefitinib combined with chemotherapy
Single-agent EGFR TKI treatment has become a standard first-line strategy in patients with advanced EGFR-mutant NSCLC, but it was hypothesised that adding chemotherapy might improve outcomes further. The phase III NEJ009 trial tested the combination of carboplatin plus pemetrexed with gefitinib compared to gefitinib alone in a total of 342 patients with previously untreated, stage IIIB/IV, non-squamous, EGFR-mutant NSCLC [3]. In the experimental arm, gefitinib plus chemotherapy was administered for 4 to 6 cycles, followed by maintenance with gefitinib plus pemetrexed until progression. Patients in the control arm received gefitinib continuously; when they progressed, the protocol recommended switching to a platinum-based regimen.
Compared with gefitinib monotherapy, the combination gave rise to significantly superior PFS (20.9 vs. 11.2 months; HR, 0.490; p < 0.001). No significant difference was seen for PFS2, which was defined as the comparison of the time to second progression in the control arm with the time to first progression in the experimental arm. Nevertheless, the combination also provided significantly prolonged OS (50.9 vs. 38.8 months; HR; 0.72; p = 0.02). ORR was higher with the combination (84.0 % vs. 68.0 %). The assessment of the clinical status at the first and second disease progression indicated that the patient performance status was better in the combination arm than in the monotherapy arm when the planned regimen failed.
Not surprisingly, haematological toxicities occurred more commonly in the combination arm, although few patients discontinued treatment due to toxicities in both arms (11.2 % vs. 9.4 %). A quality-of-life analysis suggested no difference across the two groups in the course of the study. The investigators stated that gefitinib combined with carboplatin and pemetrexed is an effective option for first-line treatment of patients with advanced EGFR-mutant NSCLC.
Final analysis of LUX-Lung 8: afatinib in squamous NSCLC
Based on the open-label, phase III LUX-Lung 8 trial, afatinib has been approved for the treatment of patients with stage IIIB/IV lung cancer of squamous histology who have progressed on or after platinum-based chemotherapy. The primary analysis of LUX-Lung 8 that compared afatinib with erlotinib showed significant improvements in the experimental arm with regard to PFS (2.6 vs. 1.9 months; HR, 0.81; p = 0.0103) and disease control rate (50.5 % vs. 39.5 %; p = 0.002) [4]. PFS and OS benefits appeared even greater for patients with ErbB-mutation–positive tumours compared to ErbB wild-type tumours [5].
The final analysis of the LUX-Lung 8 trial that was presented at the ESMO 2018 Congress confirmed these results [6]. Updated OS was significantly longer with afatinib than with erlotinib (7.8 vs. 6.8 months; HR, 0.84; p = 0.0193). Twenty-one patients in the experimental arm had long-term disease control (≥ 12 months’ treatment). In this group, certain genetic aberrations, particularly in the ErbB family, were more common than in the overall afatinib-treated population. These patients were on treatment for a median of 19.0 months; their median PFS and OS amounted to 12.9 and 27.5 months, respectively. Partial responses were achieved in 29 %.
Long-term treatment was well tolerated, with a predictable tolerability profile that was manageable with supportive care and tolerability-guided dose reductions. According to the conclusion of the authors, these data position afatinib as a treatment option for patients with squamous-cell carcinoma of the lung progressing on chemotherapy, particularly those with ErbB family genetic aberrations.
GIDEON & NEJ027
Brueckl et al. reported the first interim analysis of GIDEON, a prospective non-interventional study that was conducted in Germany to investigate the activity and tolerability of first-line afatinib in routine clinical care [7]. Among 151 treated patients, the majority (72.8 %) started treatment at an afatinib dose of ≥ 40 mg; 61.8 % of these had dose reductions. In the group of patients starting at < 40 mg, 46.2 % had dose reductions, while dose increases were performed in 33.3 %. The safety profile of afatinib was consistent with the known safety profile identified by the clinical trials.
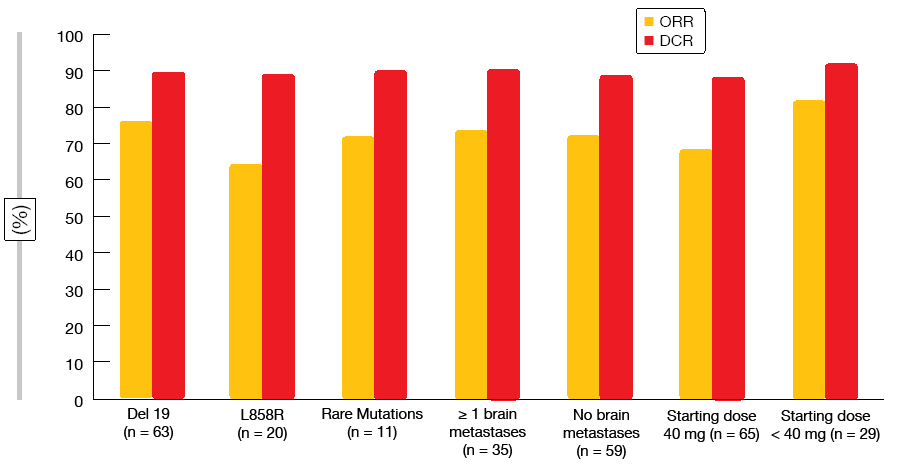
In spite of relatively high proportions of patients with brain metastases (approximately 30 %) and uncommon EGFR mutations (approximately 13 %), the results corroborated the clinical data for afatinib in the routine setting. Median PFS was 12.9 months, with a 12-month PFS rate of 54.6 %. Seventy-three percent of patients responded, and 90 % obtained disease control. Both ORRs and disease control rates (DCR) were independent of the type of EGFR mutation, the presence of baseline brain metastases, and starting dose (Figure 2). Afatinib proved efficacious in the elderly population that is underrepresented in clinical trials. Patients aged < 75 years and ≥ 75 benefited equally from the treatment with regard to median PFS (12.2 and 14.2 months, respectively). The preliminary OS analysis revealed an overall median OS of over 33 months. Final results of the GIDEON trial are expected for 2019.
Likewise, the open-label, single-arm, phase II NEJ027 study established the efficacy and safety of afatinib in elderly patients (≥ 75 years) with EGFR-mutant advanced NSCLC (n = 37) [8]. ORR and DCR were 75.7 % and 89.2 %, respectively. Median PFS was 14.3 months, with 64.3 % of patients showing freedom from progression at 1 year. OS follow-up is ongoing; the 1-year survival rate amounted to 83.6 %. Dose reductions and temporary withdrawal became necessary in 78.9 % and 73.7 %, respectively. Overall, the patients were treated for 368.0 days at a mean daily dose of 28.4 mg.
Figure 2: Overall response rates and disease control rates obtained with first-line afatinib in the non-interventional GIDEON study
Osimertinib: resistance data from FLAURA …
The third-generation, CNS-active EGFR TKI osimertinib has shown superior efficacy compared with gefitinib and erlotinib as first-line treatment in patients with advanced EGFR-mutated NSCLC in the phase III FLAURA study [9]. Published data concerning the mechanisms of acquired resistance to first-line osimertinib are limited to date. However, increased understanding is essential here to inform future therapeutic development. At the ESMO 2018 Congress, Ramalingam et al. presented candidate mechanisms of acquired resistance to first-line osimertinib detected in plasma samples from patients who progressed or discontinued treatment during FLAURA [10]. The analysis focussed on genomic alterations detectable in circulating tumour DNA (ctDNA). Non-genetic mechanisms of resistance, including SCLC transformation and protein expression changes, were not captured. Also, amplification events might be underrepresented in plasma analyses.
Paired plasma samples obtained at baseline and at the time of progression or discontinuation were analysed used next-generation sequencing (NGS). Among 272 patients with paired NGS data, 129 and 91 who were treated with the comparator EGFR TKIs and osimertinib, respectively, had EGFR mutations in their baseline plasma samples and therefore were included in the analysis. This showed that the most common acquired resistance mechanism in the comparator-treated group was, as expected, the EGFR T790M mutation (47 %). Furthermore, MET amplification (4 %), and HER2 amplification (4 %) were present. PIK3CA mutations occurred in 3 %. Two percent of patients developed RET fusion gene abnormalities.
With osimertinib treatment, no patient showed evidence of T790M-mediated acquired resistance. The most common mechanisms included MET amplification (15 %) and EGFR C797S mutation (7 %). Three percent of patients developed other secondary EGFR mutations, such as L718Q. PIK3CA mutations occurred in 7 %, HER2 amplification in 2 %, HER2 mutation in 1 %, and BRAF and KRAS mutations in 3 % each. Various alterations in the cell-cycle–related genes were observed in a total of 10 %. Approximately 14 % of the patients had concurrent candidate resistance mutations, which indicates that more than one pathway is involved in the development of resistance.
Overall, these results did not suggest new mechanisms of osimertinib resistance in the first-line treatment setting that give rise to aggressive disease biology. However, tissue-based testing is required to understand the full spectrum of resistance aberrations. Ongoing research will therefore address tissue analysis for mechanisms of resistance to first-line osimertinib.
… and AURA3
Similar data on acquired resistance mechanisms in osimertinib-treated patients have been obtained from the randomised AURA3 trial [11]. AURA3 established the superiority of osimertinib over chemotherapy in T790M-positive advanced NSCLC following progression on first-line EGFR TKI therapy [12]. The plasma ctDNA genomic profile was investigated in patients who progressed on osimertinib treatment during the AURA3 trial, with a focus on acquired mutations. As for the FLAURA analysis, paired plasma samples from baseline and the time of progression/discontinuation were collected and assessed using NGS. Seventy-three and 24 patients in the osimertinib and chemotherapy arms, respectively, were included. Again, the analysis did not capture non-genetic mechanisms of resistance, and most likely it did not fully reflect amplification events.
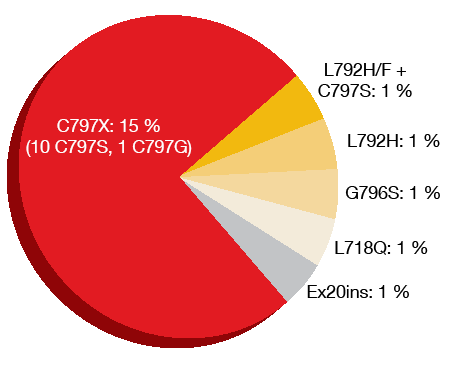
Loss of the T790M resistance mutation had occurred in 49 % of samples at the time of progression/discontinuation, which was consistent with previous studies [13-15]. It has been observed that the elimination of T790M-harbouring clones frequently co-occurs with the emergence of other competing resistance mechanisms. Acquired EGFR mutations, most commonly the C797S mutation, were seen in 21 % of patients (Figure 3). All of the patients with acquired EGFR mutations retained T790M. MET amplification was found in approximately 19 % and co-occurred with EGFR C797S mutation (7 %) as well as EGFR G796S mutation and HER2 amplification (1 %). Both T790M loss and preservation prevailed in MET-amplified samples. Cell cycle gene alterations emerged in 12 %. HER2 amplifications were identified in 5 % of patients, oncogenic fusions in 3 %, and BRAFV600E mutations in 3 %. The analysis yielded more than one resistance-related alteration in 19 % of patients.
Progression-free survival was assessed preliminarily according to the candidate resistance mechanisms. However, due to the heterogeneity of these, the numbers for each event were small. Loss of T790M showed a correlation with slightly shorter median PFS (5.54 months) compared to preservation of T790M (7.06 months).
The authors noted in their conclusion that the overlap of targetable alterations has clinical implications when determining subsequent treatments. Research into the novel mechanisms of resistance to osimertinib and appropriate therapeutic strategies is ongoing. For instance, a prospective single-arm phase II study will assess the combination of afatinib and bevacizumab in patients after osimertinib failure [16]. It is hypothesised that this regimen might overcome resistance mechanism implicated in osimertinib failure, including uncommon EGFR mutations and MET amplification.
Figure 3: Acquired EGFR mutations after osimertinib treatment in AURA3
First-line nazartinib: phase II results
Like osimertinib, nazartinib is an oral third-generation EGFR TKI that selectively targets activating EGFR mutations as well as resistant mutants such as T790M while sparing wild-type EGFR. A phase I/II, multicentre study conducted in patients with advanced EGFR-mutant NSCLC who had received ≤ 3 prior lines of systemic therapy established 150 mg once daily as the recommended phase II dose [17]. Preliminary results of the phase II part of the study in treatment-naïve patients showed promising efficacy despite a high proportion of patients with brain metastases at baseline [18]. At the ESMO 2018 Congress, Tan et al. reported the primary efficacy and safety findings obtained with nazartinib 150 mg daily as first-line therapy until progression in 45 patients with EGFR-mutant, locally advanced or metastatic NSCLC [19]. Treated and stable brain metastases were allowed.
The confirmed ORR by blinded independent review was 64.4 % including one confirmed complete response and 28 confirmed partial responses. Disease control resulted in 93.3 %. Most of the patients experienced reductions in the size of their target lesions. PFS and duration of response data were still immature at the time of data cut-off.
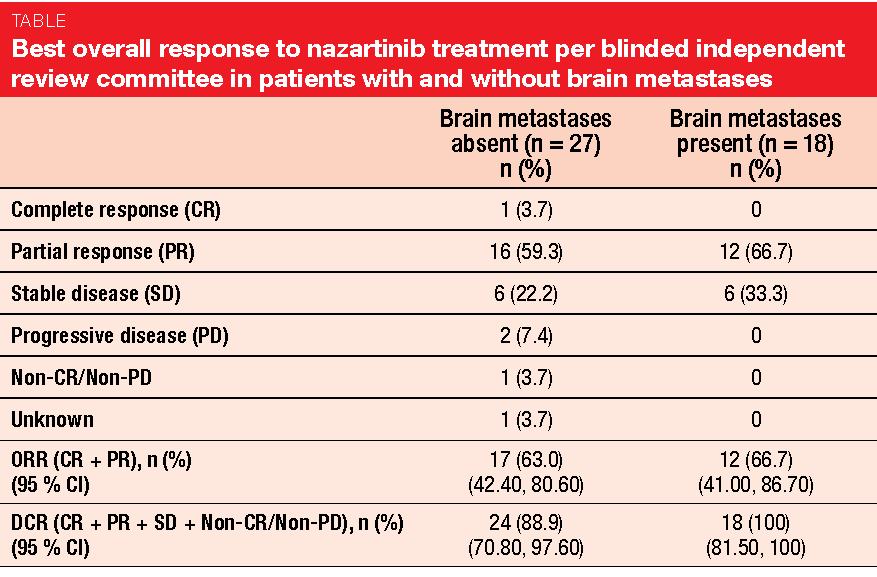
Moreover, nazartinib showed activity in patients with CNS lesions at baseline. Confirmed ORRs were 66.7 % and 63 % in those with and without brain metastases, respectively, and DCRs were 100 % and 88.9 %, respectively (Table). Among 18 patients with baseline brain lesions, absence or normalisation of lesions was observed in 52.9 % of those with brain non-target lesions. Patients with brain target lesions obtained a 38.5 % decrease in size from baseline. Only two patients experienced cerebral progression with new CNS metastases. Nazartinib showed a tolerable safety profile.
REFERENCES
- Zhong W et al., Phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 non-small cell lung cancer based on epidermal growth factor receptor mutation status. J Hematol Oncol 2015; 8: 54
- Zhong WZ et al., Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment for stage IIIA-N2 EGFR-mutation positive non-small-cell lung cancer (EMERGING-CTONG 1103): multicentre phase 2 randomized study. ESMO 2018, abstract LBA48_PR
- Seike M et al., Phase III study of gefitinib (G) versus gefitinib + carboplatin + pemetrexed (GCP) as 1st-line treatment for patients with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). ESMO 2018, abstract 1382PD
- Soria JC et al., Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015; 16(8): 897-907
- Goss GD et al., Association of ERBB Mutations With Clinical Outcomes of Afatinib- or Erlotinib-Treated Patients With Lung Squamous Cell Carcinoma: Secondary Analysis of the LUX-Lung 8 Randomized Clinical Trial. JAMA Oncol 2018; 4(9): 1189-1197
- Goss GD et al., Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung: final analysis of the global phase III LUX-Lung 8 trial. ESMO 2018, abstract 1442P
- Brueckl WM et al., Effectiveness of afatinib in clinical practice – first results of the GIDEON trial: a prospective non-interventional study in EGFR-mutated NSCLC in Germany. ESMO 2018, abstract 1449P
- Aiba T et al., A phase II study of first-line afatinib for patients aged 75 or older with EGFR mutation-positive advanced non-small cell lung cancer: North East Japan Study Group Trial NEJ027. ESMO 2018, abstract 1445P
- Soria JC et al., Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113-125
- Ramalingam SS et al., Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the double-blind, randomised phase III FLAURA study. ESMO 2018, abstract LBA50
- Papadimitrakopoulou V et al., Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. ESMO 2018, abstract LBA51
- Mok TS et al., Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017; 376: 629-640
- Oxnard GR et al., Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol 2018 Aug 2. doi: 10.1001/jamaoncol.2018.2969. [Epub ahead of print]
- Lin CC et al., Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic study. Lancet Res Med 2018; 6(2): 107-116
- Le X et al., Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy post-progression in EGFR-mutant NSCLC. Clin Cancer Res 2018 Sep 18. pii: clincanres.1542.2018. doi: 10.1158/1078-0432.CCR-18-1542. [Epub ahead of print]
- Hata A et al., Afatinib plus bevacizumab combination after osimertinib failure for advanced EGFR-mutant non-small cell lung cancer: a multicentre prospective single arm phase II study (ABCD study). ESMO 2018, abstract 1501TiP
- Tan DS et al., Updated results of a phase 1 study of EGF816, a third-generation, mutant-selective EGFR tyrosine kinase inhibitor (TKI), in advanced non-small cell lung cancer (NSCLC) harboring T790M. J Clin Oncol 34, 2016 (suppl; abstr 9044)
- Kim DW et al., Preliminary Phase II results of a multicenter, open-label study of nazartinib (EGF816) in adult patients with treatment-naïve EGFR-mutant non-small cell lung cancer (NSCLC). J Clin Oncol 36, 2018 (suppl; abstr 9094)
- Tan DSW et al., Phase II results for single-agent nazartinib (EGF816) in adult patients (Pts) with treatment-naïve EGFR-mutant non-small cell lung cancer (NSCLC). ESMO 2018, abstract LBA61