Frontline combinations of EGFR- and angiogenesis-targeted agents
In patients with untreated EGFR-mutant tumors, it has been shown that the addition of the anti-VEGF antibody bevacizumab to first-generation EGFR TKIs induces PFS benefits with an acceptable toxicity profile [1, 2]. The open-label, randomized, multicenter, phase III ARTEMIS (CTONG 1509) study is the first phase III trial to test bevacizumab plus erlotinib in Chinese NSCLC patients [3]. At 14 sites in China, a total of 311 patients with EGFR-mutated (i. e., exon 19 deletion or exon 21 L858R mutation), advanced NSCLC received either bevacizumab plus erlotinib (n = 157) or erlotinib (n = 154) until progression. PFS according to independent review committee (IRC) constituted the primary endpoint.
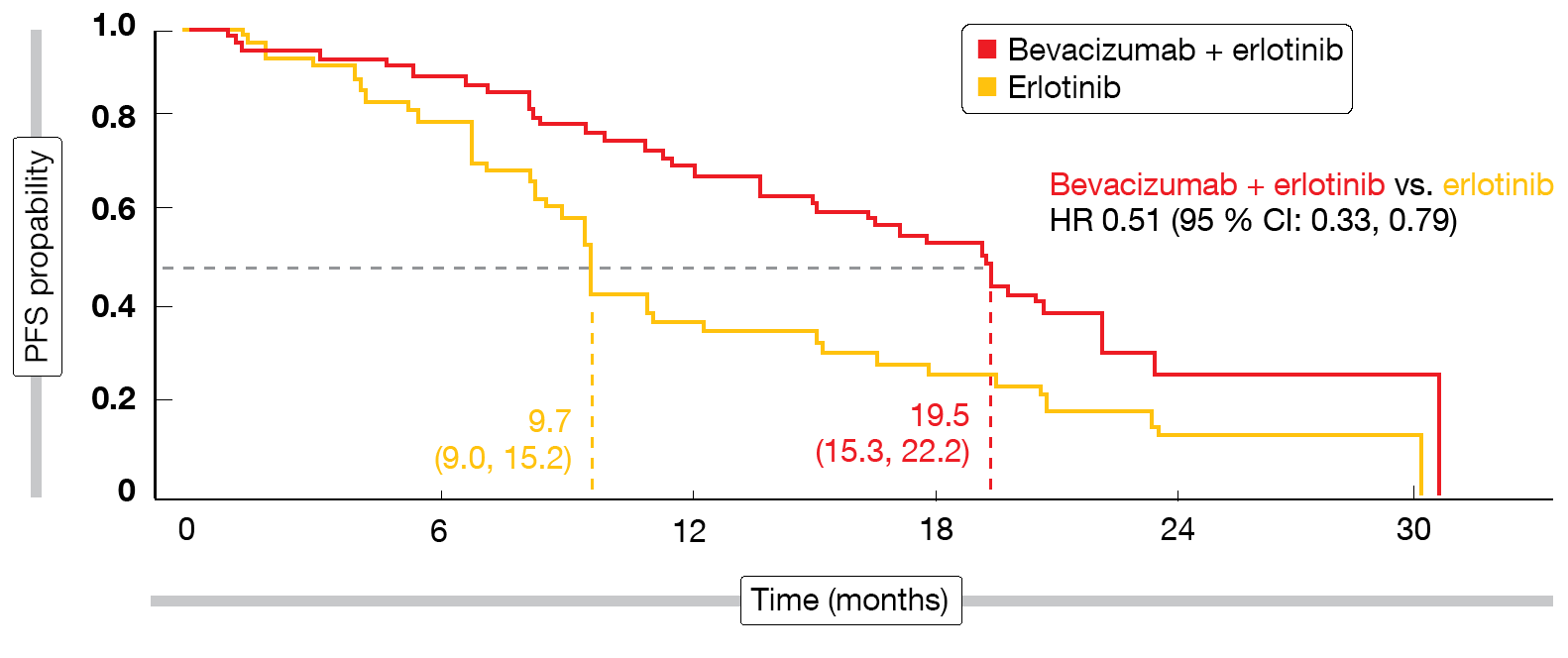
PFS difference of up to 10 months
The addition of bevacizumab indeed induced statistically significant and clinically relevant PFS improvement (18.0 vs. 11.3 months; HR, 0.55; p < 0.001). Subgroup analyses showed that patients with L858R mutations and those with brain metastases at baseline appeared to derive particular PFS benefit from the combined approach. For the group with L858R mutations that made up approximately half of the total cohort, median PFS was 19.5 vs. 9.7 months by IRC (HR, 0.51; Figure), thus exceeding the PFS findings observed for deletion 19 (17.9 vs. 12.5 months; HR, 0.62). Response rates were generally high and did not differ across treatment arms. ORRs were 86.3 % vs. 84.7 % according to IRC, and disease control occurred in 95.9 % vs. 96.5 %. However, duration of response was longer with bevacizumab plus erlotinib than with erlotinib alone (16.6 vs. 11.1 months according to IRC; HR, 0.59). Adverse events related to the combination therapy proved tolerable and manageable. No new safety signals were detected.
The study included a resistance biomarker analysis that involved testing of tissue samples using next-generation sequencing and transcriptome sequencing. At the time of progression, the EGFR T790M resistance mutation was identified less frequently in the combination arm than with erlotinib (33 % vs. 42 %). Also, patients in the experimental arm showed a smaller proportion of new mutations and amplifications, which implies different resistance mechanisms. The authors concluded that bevacizumab plus erlotinib is expected to become the new first-line standard for the treatment of advanced, EGFR-mutant NSCLC.
Figure: Progression-free survival benefit with bevacizumab plus erlotinib observed in patients harboring exon 21 L858R mutations
Resistance mechanisms in RELAY
Another antiangiogenic agent investigated as a combination partner of EGFR-targeted agents is the anti-VEGF-2 antibody ramucirumab. In the global, phase III RELAY trial, patients with EGFR-mutation–positive NSCLC were randomized to either ramucirumab combined with erlotinib or placebo plus erlotinib. Compared to erlotinib monotherapy, the combination improved median PFS to a significant extent (19.4 vs. 12.4 months; HR, 0.591; p < 0.0001) [4]. An exploratory Japanese substudy of RELAY focused on the occurrence and clinical effect of the T790M mutation as an acquired resistance mechanism [5]. EGFR mutations were determined using ctDNA from plasma samples collected before treatment, during treatment, and after disease progression. The biomarker-evaluable population included 65 individuals.
According to this analysis, post-progression T790M mutation rates did not differ between treatment groups despite the PFS advantage conferred by the combination. T790M rates, when analyzed according to the number of treatment cycles before progression, were comparatively lower with ramucirumab plus erlotinib than with erlotinib, although not significantly so. Thus, the combination might delay the occurrence of resistance due to T790M mutations. PFS was not markedly affected by the presence or absence of the T790M mutation in either treatment group. Post-progression T790M rates detected by droplet digital polymerase chain reaction corresponded to those in the overall intent-to-treat population detected by next-generation sequencing, suggesting the potential for effective EGFR-directed therapy after progression on ramucirumab plus erlotinib.
Bevacizumab plus afatinib
Based on the hypothesis that the combination of bevacizumab with the second-generation EGFR TKI afatinib might improve efficacy, the phase I Okayama Lung Cancer Study Group Trial 1404 assessed afatinib plus bevacizumab as frontline treatment in 19 patients with advanced EGFR-positive NSCLC. Evidence of disease control including responses in 13 out of 16 evaluable patients has been reported in 2018 [6]. Ninomiya et al. presented the secondary endpoints, which included response rate, PFS, OS and toxicity, at ESMO 2019 [7].
After a median follow-up of 27.4 months, PFS was 24.2, months, and median OS had not been reached yet. The PFS findings did not differ according to the type of EGFR mutation (24.2 and 23.8 months for deletion 19 and L858R mutation, respectively). However, patients with ECOG PS 0 showed significantly longer PFS than those with PS 1 (not reached vs. 13.4 months; p = 0.0192). ORR amounted to 81.3 % in the total population, with complete responses occurring in 6.3 %.
At two years, seven patients were still on treatment, while five had discontinued due to disease progression, four due to toxicity, and three based on their preference. Rebiopsies showed the presence of T790M mutation at the time of progression in two cases, and osimertinib was prescribed. Among adverse events, acneiform rash, diarrhea, paronychia, proteinuria and hypertension occurred most commonly. No grade ≥ 4 adverse events were observed. A randomized trial comparing afatinib plus bevacizumab with single-agent afatinib is ongoing.
REFERENCES
- Ichihara E et al., Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol 2015; 10(3): 486-491
- Seto T et al., Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014; 15(11): 1236-1244
- Zhou Q et al., ARTEMIS (CTONG 1509): phase 3 study of bevacizumab with or without erlotinib in untreated Chinese patients with advanced EGFR-mutated NSCLC. WCLC 2019, abstract 1480O
- Nakagawa K et al., RELAY: A multinational, double-blind, randomized Phase 3 study of erlotinib in combination with ramucirumab or placebo in previously untreated patients with epidermal growth factor receptor mutation-positive metastatic non-small cell lung cancer. J Clin Oncol 37, 2019 (suppl; abstr 9000)
- Nishio K et al., Impact of ramucirumab + erlotinib on EGFR mutations in circulation tumor DNA – the 1st report of a biomarker study in Japanese patients from RELAY: global phase 3 study of erlotinib + ramucirumab or placebo in 1L metastatic NSCLC with EGFR activating mutations. ESMO 2019, abstract 1523P
- Ninomiya T et al., A phase I trial of afatinib and bevacizumab in chemo-naïve patients with advanced non-small-cell lung cancer harboring EGFR mutations: Okayama Lung Cancer Study Group Trial 1404. Lung Cancer 2018; 115: 103-108
- Ninomiya T et al., Updated analysis of a phase I trial of afatinib and bevacizumab in chemo-naïve patients with advanced non-small-cell lung cancer harboring EGFR mutations: OLCSG1404. ESMO 2019, abstract 1525P