Chemotherapy: new approaches, new settings
SCAT: customising adjuvant chemotherapy using BRCA1
Current guidelines recommend postoperative platinum-based chemotherapy in completely resected NSCLC with nodal involvement (stage II-IIIA) [1]. However, survival outcomes remain limited, and compliance is lower than for adjuvant therapy in other neoplasms. There are no direct comparisons between different chemotherapy regimens.
The analysis of expression of genes involved in DNA repair could be used to individualise the choice of optimal chemotherapy agents and schedules [2]. Here, the BRCA1 gene has significance as it plays a role in the homologous recombination pathway and functions as a differential regulator of response to cisplatin and antimicrotubule agents. It has prognostic and predictive relevance; low levels translate into low risk and cisplatin sensitivity, while high levels indicate high risk and cisplatin resistance, which implies that the patient is sensitive to taxane-based chemotherapy.
A BRCA1-guided treatment approach was tested by the randomised SCAT trial, which contained patients with resected NSCLC R0 pN1/ pN2 [3]. While the control arm received docetaxel plus cisplatin, patients randomised to the experimental arm were treated according to BRCA1 expression levels. Patients with low BRCA1 expression received gemcitabine/ cisplatin, those with medium levels, cisplatin/ docetaxel, and those with high levels, docetaxel alone. Four cycles were administered every 21 days. Chemotherapy was started within 8 weeks after surgery. The per-protocol treatment population included 102 patients in the control arm and 354 in the experimental arm. OS constituted the primary endpoint.
Single-agent docetaxel appears sufficient in high expressors
Low levels of BRCA1 expression were significantly associated with female sex, never-smoking status, adenocarcinoma histology, and mediastinal lymph node involvement. Higher levels, on the other hand, correlated with male sex, squamous histology, and current or former smoker status.
According to the primary analysis, customisation of adjuvant chemotherapy according to BRCA1 levels did not induce a significant OS difference between the experimental arm and the control arm (82.4 vs. 69.3 months; HR, 0.946). Five-year survival rates exceeded 50 % in both arms (56 % and 54 %, respectively). In the experimental group, there was no striking variation of median OS, which ranged from 74 to 80.5 months. In contrast, patients treated in the control cohort fared worst when expressing high BRCA1 levels (OS, 40.1 months), whereas outcomes were markedly improved for those with intermediate and high levels (not reached and 82.4 months, respectively). In a multivariate Cox analysis, BRCA1 levels were found to be prognostic in the control group.
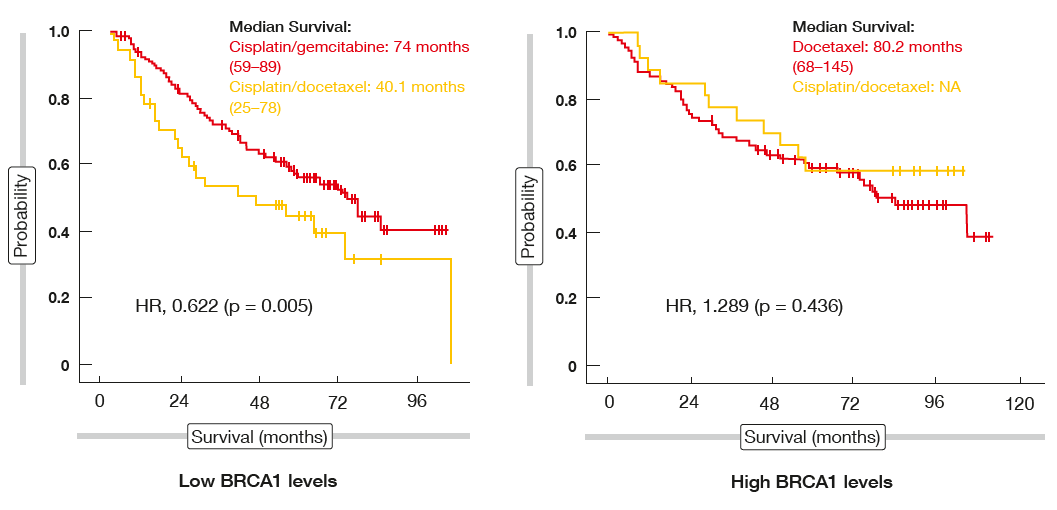
When analysed across the two treat-ment arms according to BRCA1 sub-group, patients with low expression levels were shown to benefit from cisplatin/ gemcitabine compared to cisplatin/ docetaxel (74 vs. 40.1 months; HR, 0.622; Figure). However, for the BRCA1 high expression group, there was no difference between the experimental and control regimens, i.e. survival achieved with docetaxel alone resembled the OS outcomes in the docetaxel/ cisplatin co-hort. The compliance relating to planned treatment was significantly improved for the group without cisplatin in the experimental arm. Patients treated without cisplatin showed a trend to lower non–cancer-related mortality. Overall, the authors concluded that adjuvant taxane treatment without a platinum component might be assessed in patients with high BRCA1 expression levels. Here, it should be possible to avoid short-term and long-term platinum toxicity.
Figure: SCAT trial: progression-free survival analysis according to BRCA1 subgroups across treatment arms
Surprisingly good second-line activity of nab-paclitaxel
Efficacious and tolerable chemotherapy options are called for in the second-line setting of advanced NSCLC. The ran-domised, open-label, multicentre phase II ABOUND.2L+ trial compared single-agent nab-paclitaxel with nab-paclitaxel plus oral azacitidine (CC-486) in 161 patients with advanced non-squamous NSCLC who had already undergone one platinum-based chemotherapy, but no prior taxane treatment [4]. Eighty patients received nab-paclitaxel monotherapy at a dose of 100 mg/m2 on days 1 and 8 of a 21-day cycle, while 80 were treated with the combination of nab-paclitaxel (days 8 and 15 of a 21-day cycle) and CC-486 (200 mg orally on days 1 to 14 of a 21-day cycle).
The study did not meet its primary endpoint, as nab-paclitaxel plus CC-486 did not demonstrate superiority regarding PFS. Patients in the control arm fared surprisingly well, experiencing even better PFS than those in the experimental arm (4.2 vs. 3.2 months; HR, 1.3). This was also true for OS (13.6 vs. 8.1 months; HR, 1.5) and ORRs (15.0 % vs. 13.6 %). Disease control, which was defined as the combination of CR, PR and SD, occurred in 67.5 % vs. 65.4 %. According to the quality-of-life analysis, nab-paclitaxel gave rise to improved outcomes for respiratory symptom, symptom burden index, and global quality-of-life scores. Both regimens were well tolerated. Grade ≥ 3 adverse events (AEs) remained in the single-digit range for both arms.
After all patients had been recruited, the investigators were advised to discontinue CC-486 treatment. Although the combination had not brought about any added benefit, single-agent nab-paclitaxel showed promise as a second-line drug in the treatment of advanced non-squamous NSCLC. Results from ongoing trials will provide further in-sight into the role of nab-paclitaxel in this setting.
Immunotherapy plus chemotherapy
A third treatment arm was implemented in the ABOUND.2L+ trial in March 2016, with the objective of investigating the addition of the anti-PD-L1 antibody durvalumab to nab-paclitaxel [5]. Seventy-nine patients with advanced non-squamous or squamous NSCLC received nab-paclitaxel 100 mg/m2 on days 1 and 8 of a 21-day cycle plus durvalumab 1,125 mg on day 15 of a 21-day cycle. Approximately one third showed squamous histology. As with the other two arms of ABOUND.2L+, one prior platinum-based chemotherapy was allowed, while prior taxanes were not, but patients could have had immune checkpoint inhibitor therapy before trial inclusion. This was the case for 11.4 % of the population. PFS was defined as the primary endpoint.
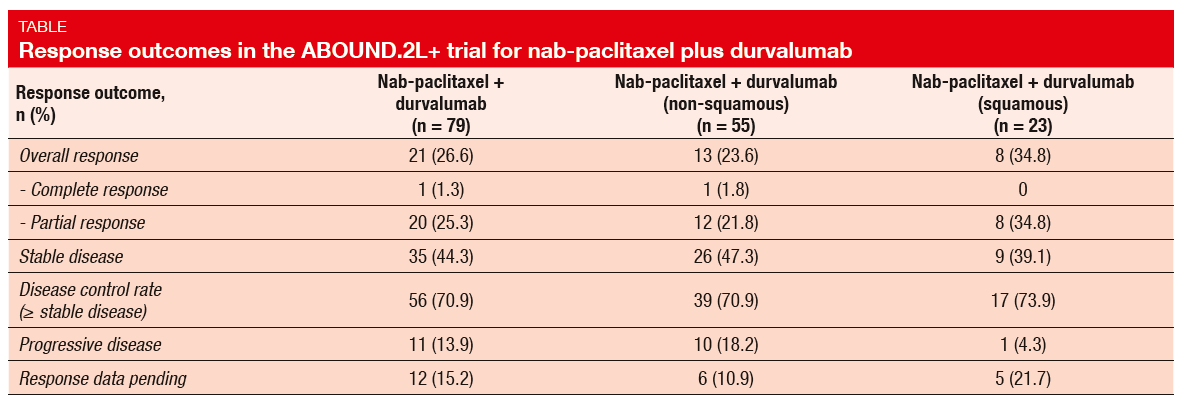
The combined treatment with paclitaxel plus durvalumab gave rise to a median PFS of 4.5 months. Median OS had not been reached yet. Somewhat unexpectedly, patients who had received immune checkpoint inhibitor treatment before enrolment experienced superior PFS compared with the checkpoint-inhibitor–naïve group (6.9 vs. 4.4 months), but these results must be regarded with caution due to the small number of pretreated patients. Also, patients with squamous histology achieved longer PFS than those with non-squamous histology (5.9 vs. 4.2 months). ORR was 26.6 % in the overall population (Table), which compares favourably to outcomes achieved with other therapies in the second-line setting. Again, the subgroup analysis revealed comparatively better findings in patients with squamous NSCLC than in those with non-squamous tumours (34.8 % vs. 23.6 %). Overall, the trial yielded a commendable DCR of 70.9 %.
Toxicity proved predictable, with peripheral sensory neuropathy, dyspnoea, neutropenia and anaemia reported as the most common AEs. Febrile neutropenia did not occur. The authors concluded that the combination of nab-paclitaxel and durvalumab demonstrated anti-tumour activity with manageable toxicity in the second-line or third-line treatment of patients with advanced NSCLC. These data provide further support for the use of nab-paclitaxel as a chemotherapy partner for immune checkpoint inhibitors in NSCLC.
Nab-paclitaxel in squamous-cell carcinoma
As therapeutic options for squamous-cell lung cancer remain limited, the phase II trial presented by Paik et al. is currently testing nab-paclitaxel plus gemcitabine in patients with untreated stage IV squamous NSCLC. The findings presented at the WCLC showed that nab-paclitaxel plus gemcitabine has promising efficacy and is well tolerated compared to platinum-based regimens [6]. Twenty-one patients were enrolled and treated with one of two dosing regimens.
ORR is defined as the primary objective of the trial. At the time of the analysis, this was 58 %, with a duration of response of 7.5 months. PFS was 6.1 months, and OS was 13.9 months. In comparison, platinum-based chemotherapies, which have been the standard first-line agents for almost 20 years, are known to induce ORRs of 30 % to 40 %, median PFS of 4 to 5.7 months, and median OS of 9 to 11.5 months [7-9]. Fatigue, oedema, peripheral neuropathy and nausea predominated among AEs, the majority of which were grade 1. Serious AEs included leukopenia, diar-rhoea, and lung infection. Accrual to the trial is ongoing with a focus on PD-L1–negative patients.
Adjuvant doublet chemotherapy including nedaplatin
Nedaplatin is a cisplatin derivative developed in Japan. A prospective, multi-institutional phase II study evaluated the feasibility of combination chemotherapy with docetaxel and nedaplatin in the adjuvant treatment of 34 patients with NSCLC stage IB-IIIA, who had undergone radical surgery including lobectomy and lymph node dissection [10]. On day 1 of 4 cycles, docetaxel and nedaplatin were administered at 60 mg/m² and 80 mg/m², respectively. Feasibility (i.e., the proportion of patients who completed 4 cycles) was defined as the primary endpoint, and toxicity and relapse-free survival (RFS) constituted the secondary endpoints.
The results demonstrated that adjuvant chemotherapy with docetaxel plus nedaplatin is feasible and tolerable for patients with completely resected NSCLC. Overall, 76.5 % of patients completed all of the 4 cycles. Median RFS had not been reached at the time of the analysis, and the 5-year RFS rate was 65.8 %. The incidence of haematologic and non-haematologic AEs was lower than for the combination chemotherapy of cisplatin plus vinorelbine tested in the ANITA trial [11].
REFERENCES
- Postmus PE et al., Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28 (Suppl 4): iv1-iv21
- Karachaliou N et al., Using genetics to predict patient response to platinum-based chemotherapy, Exp Rev Prec Med Drug Develop 2017; 2(1): 21-32
- Massuti B et al., SCAT phase 3 trial: adjuvant CT based on BRCA1 levels in NSCLC N+ resected patients. Final survival results. A Spanish Lung Cancer Group trial. WCLC 2017, PL 02.04
- Morgensztern D et al., nab-Paclitaxel ± CC-486 as second-line treatment of advanced NSCLC: results from the ABOUND.2L+ study. WCLC 2017, MA 03.01
- Govindan R et al., nab-paclitaxel + durvalumab as second- or third-line treatment ad advanced NSCLC: results from ABOUND.2L+. WCLC 2017, MA 03.01
- Paik PK et al., A phase II trial of albumin-bound paclitaxel and gemcitabine in patients with untreated stage IV squamous cell lung cancers. WCLC 2017, P1.03-028
- Scagliotti GV et al., Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26(21): 3543-3551
- Socinski MA et al., Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012; 30(17): 2055-2062
- Thatcher N et al., Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cispl-atin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015; 16(7): 763-774
- Teramoto K et al., A phase II study of adjuvant chemotherapy with docetaxel plus nedaplatin for completely resected non-small-cell lung cancer. WCLC 2017, P1.03-37
- Douillard JY et al., Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006; 7(9): 719-727