Advances in PSMA radiotracers for prostate cancer imaging
[18F]DCFPyL tops [18F]Flurocholine in the recurrent setting: PYTHON
Prostate-specific membrane antigen (PSMA)-targeting positron emission tomography/computed tomography (PET/CT) imaging is increasingly used to characterize prostate cancer (PCa). However, in Europe, there is still an unmet need for radiotracers to localize biochemical recurrences in PCa. The phase III PYTHON trial is designed to establish the efficacy and safety of [18F]DCFPyL- compared to [18F]Flurocholine-PET/CT in patients with first biochemical recurrence after initial definitive therapy (prostatectomy, external beam radiotherapy or brachytherapy) for histopathologically confirmed prostate adenocarcinoma per original diagnosis [1]. The primary objective is the per-patient detection rate (DR) of both tracers, while the secondary objective includes the assessment of the impact on patient treatment management, the per-region detection rate, the sensitivity and specificity on a per-patient and per-region basis, the concordance rate between both tracers, and the safety.
A total of 205 patients were enrolled in the study, with 73.2 % undergoing radical prostatectomy as initial treatment and 38.7 % belonging to a high D’Amico risk class. DR of [18F]DCFPyL-PET/CT was higher than [18F]Flurocholine-PET/CT (58.2 % vs. 40.3 %; p < 0.0001, Figure 1) and was independent of the initial treatment or prostate-specific antigen (PSA) detection, D’Amico risk class, or International Society of Urologic Pathologists (ISUP) grade. Safety results were similar between the two tracers, with all treatment-emergent adverse events (TEAEs) reported being unrelated to the tracer injection. Moreover, [18F]DCFPyL-PET/CT showed a higher impact on patient treatment management.
The positive efficacy and safety results from the PYTHON trial reinforced the diagnostic performance of [18F]DCFPyL-PET/CT shown previously in its pivotal US clinical trials, OSPREY [2] and CONDOR [3], which led to its FDA approval in May 2021.
Figure 1: Detection rates for [18F]DCFPyL- and [18F]Flurocholine-PET/CT based on patient’s PSA values
18F-rhPSMA-7.3 PET informs salvage therapy decisions in recurrent prostate cancer: additional results from Phase 3 SPOTLIGHT Study
18F-rhPSMA-7.3 – a radiohybrid (rh) platform – represents a new class of high-affinity PSMA-targeted theranostic PET radiopharmaceuticals with the potential for low bladder activity being investigated for diagnostic imaging in patients with prostate cancer. The phase III SPOTLIGHT trial included patients with suspected prostate cancer recurrence based on elevated PSA following prior therapy, and being eligible for salvage therapy with curative intent. Patients were imaged with 296 MBq of 18F-rhPSMA-7.3, followed by PET/CT 50–70 minutes post-injection. Three blinded central readers evaluated the scans [4].
A clinically significant 57 % Standard of Truth (SoT)-verified DR was presented at ASCO GU 2022, where composite SoT consisting of either histopathology or conventional imaging was used to confirm positive findings [4].
At EANM 2022, Hermsen et al. presented an exploratory analysis of the true positive (TP) 18F-rhPSMA-7.3 scans in pelvic lymph nodes or extra-pelvic (metastatic) regions that led to upstaging in a subgroup of patients who had presented a negative baseline conventional imaging ≤118 days before PET [5].
Of the 366 men who were scanned and had sufficient data to determine SoT, 250 (68 %) had conventional imaging indicating negative at baseline, comprised mainly of 99mTc-bone scan (56 %) or CT (46 %). According to the 3 blinded readers, TP lesions in pelvic lymph nodes were detected in 18–21 % of patients who had undergone prostatectomy and 6.5 % of patients who had received radiotherapy. The TP rates in extra-pelvic metastatic sites leading to upstaging accounted for 21–26 % of patients who had undergone prostatectomy and 20–30 % of patients who had received radiotherapy.
These data showed that investigational 18F-rhPSMA-7.3 PET/CT frequently identified TP pelvic nodal and metastatic lesions, supporting its potential clinical utility in men with recurrent prostate cancer to help define sites of disease recurrence and inform salvage therapy decisions.
Predictive benefit of PSMA- and FDG-PET in mCRPC: TheraP trial analysis
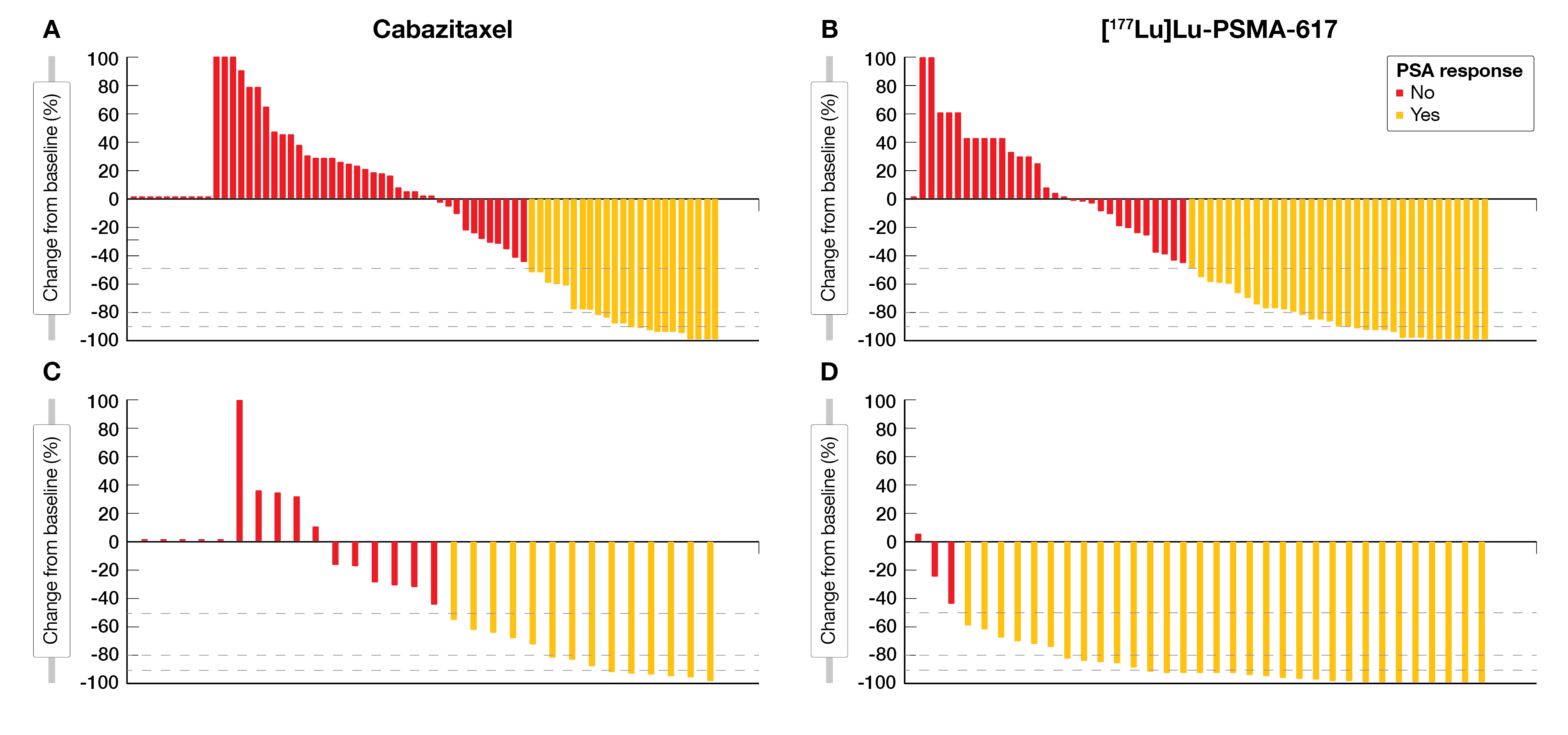
TheraP trial is the first randomized trial demonstrating that the treatment with [177Lu]Lu-PSMA-617 improves the PSA response rate by more than 50 % from baseline (PSA-50RR; 66 % vs. 37 %) and delays progression-free survival (hazard ratio (HR) 0.63; 95 % CI 0.46-0.86; p = 0.0028) compared to cabazitaxel in patients with metastatic castration-resistant prostate cancer (mCRPC) [6]. Inclusion criteria were high PSMA uptake (maximum standardized uptake value (SUVmax) ≥20) on a [68Ga]Ga-PSMA-11 PET (PSMA-PET) scan at a disease site and no [18F]fluoro-2-deoxy-D-glucose (FDG)-positive/PSMA-negative lesions. Buteau et al. presented data from a prespecified tertiary endpoint of PSMA-PET and FDG-PET imaging parameters as well as predictive and prognostic biomarkers in the TheraP trial population [7, 8].
mCRPC patients who had prior docetaxel treatment and were eligible for cabazitaxel treatment were assigned 1:1 to either cabazitaxel (20 mg/m2 IV every 3 weeks for up to 10 cycles) or [177Lu]Lu-PSMA-617 (6·0–8·5 GBq IV every 6 weeks for up to six cycles). High PSMA uptake (SUVmean of ≥10 on PSMA-PET) was evaluated as a predictive biomarker for response and metabolic tumor volume (MTV) of ≥200 mL in FDG-PET as a prognostic biomarker, respectively.
Out of the 200 randomized patients, 35 % of the [177Lu]Lu-PSMA-617 and 30 % of the cabazitaxel treatment arm had a high PSMA uptake (SUVmean ≥10) which in turn predicted a higher likelihood of favorable response to [177Lu]Lu-PSMA-617 than cabazitaxel (Odds ratio (OR) 12.2 vs. 2.2; p = 0.03). Moreover, a PSA50-RR benefit of 91 % vs. 47 % in patients with SUVmean ≥10 and 52 % vs. 32 % in patients with SUVmean <10 was reported (Figure 2).
On the other hand, high-volume disease measured by FDG-PET (MTV ≥200 mL) was noted in 30 % of patients in both treatment arms. The PSA50-RR for randomized groups combined for FDG MTV ≥200 mL vs. <200 mL were 23/60 (38 %) vs. 79/140 (56 %), respectively. After accounting for treatment, higher FDG volume was suggested as a prognostic marker for worse response to either [177Lu]Lu-PSMA-617 or cabazitaxel (OR 0.44; p = 0.01).
The authors concluded PSMA-PET SUVmean as a potential predictive biomarker for a higher likelihood of favorable response to [177Lu]Lu-PSMA-617 than cabazitaxel, providing guidance for optimal [177Lu]Lu-PSMA-617 use in mCRPC patients. High FDG-PET MTV was associated with lower responses regardless of assigned treatment, warranting further investigation for treatment intensification.
Figure 2: Waterfall plots of the best PSA decline from baseline for patients with PSMA SUVmean <10 (A, B) and PSMA SUVmean ≥10 (C, D) who were allocated cabazitaxel (A, C) vs [177Lu]Lu-PSMA-617 (B, D)
REFERENCES
- Rischpler C et al. Results from the PYTHON trial: A Prospective Study On [18F]DCFPyL-PET/CT Imaging In First Biochemical Recurrence – Prostate Cancer After Primary Therapy With Curative Intent. EANM 2022 (Oral Abstract OP-153)
- Pienta KJ et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021;206(1):52-61.
- Morris MJ et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021;27(13):3674-3682.
- Schuster DM et al. Detection rate of 18F-rhPSMA-7.3 PET in patients with suspected prostate cancer recurrence: Results from a phase 3, prospective, multicenter study (SPOTLIGHT). Journal of Clinical Oncology 2022;40, no. 6_suppl:9-9
- Hermsen R et al. Impact of 18F-rhPSMA-7.3 PET on Nodal and Metastatic Upstaging of Patients with Biochemical Recurrence of Prostate Cancer: Results from the Prospective, Phase 3, Multicenter, SPOTLIGHT Study. EANM 2022 (Oral Abstract OP-155)
- Hofman MS et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 2021; 397: 797–804
- Buteau J et al. PSMA and FDG PET as Predictive and Prognostic Biomarkers in Men with Metastatic, Castration-Resistant Prostate Cancer (mCRPC): an Analysis of the Randomised, Phase 2 Trial of [177Lu]Lu-PSMA-617 Versus Cabazitaxel (TheraP, ANZUP 1603). EANM 2022 (Oral Abstract OP-160)
- Buteau JP et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022;23(11):1389-1397
© 2023 Springer-Verlag GmbH, Impressum
More posts
Preface – EANM 2022
Preface – EANM 2022 © private - Oana C. Kulterer, MD, Division of Nuclear Medicine, D


![Figure 1: Detection rates for [18F]DCFPyL- and [18F]Flurocholine-PET/CT based on patient’s PSA values](https://memoinoncology.com/wp-content/uploads/2023/02/Picture_EANM2022_10.png)