Neuroendocrine tumor imaging updates
Little impact of interim PET/CT staging during PRRT for NETs
Guideline recommendations for peptide receptor radionuclide therapy (PRRT) using 177Lu- DOTA-0-Tyr3-Octreotate (DOTATATE) in patients with neuroendocrine tumors (NETs) include 3-5 cycles with a dose ranging from 5.5-7.4 GBq per cycle with 6-12 weeks intervals [1]. While PET with radionuclide-labeled somatostatin analogs (SSAs) is mandatory before PRRT, interim PET imaging is not routinely recommended. Since 20-30 % of patients show early disease progression (PD), a multicentric analysis of the impact of interim PET staging with SSTR-analogs after 2 cycles of 177Lu-PRRT in neuroendocrine tumor (NET) patients was conducted, and data were presented at EANM 2022 [2].
A total of 225 NET patients were included in the analysis with radiographic response in 68Ga-DOTATATE / -DOTATOC PET/CT at baseline and after two cycles of 177Lu-DOTATATE treatment. Interim PET revealed partial response (PR) in 27 (12 %), stable disease (SD) in 157 (70 %), and PD in 41 (18 %) patients. No significant difference was observed for primary tumors and the Ki-67 index between responders and non-responders. 177Lu-DOTATATE treatment was interrupted in 57 patients (25 %), with no significant difference in interim PET results between patients with and without treatment interruption. Despite PD in interim PET, treatment was continued in 19 patients (33 %). The future retrospective evaluation of this group of patients would be of interest regarding metabolic and molecular response, 18F-FDG-PET, and textural analysis.
In this large cohort study presented, interim PET/CT staging after two cycles of 177Lu-DOTATATE had a minor impact and is of limited value in the current clinical management of NET patients concerning interruption of treatment. The authors concluded that the results support continued PRRT for 4 cycles in case of good clinical tolerance.
Previous SSA treatment does not impact [18F]SiTATE-PET/CT imaging
NETs express somatostatin receptors (SSRs) and are frequently treated with SSAs as well as staged and monitored with radionuclide-labeled SSAs. Any previous treatment with SSAs could potentially reduce the sensitivity of the SSA radiotracer in PET/CT. A new SSR-targeting PET/CT imaging compound, [18F]SiTATE, has been developed for NETs [3]. Sheikh et al. presented data from a study evaluating the impact of previous long-acting SSAs treatment, in patients with differentiated gastro-entero-pancreatic NETs (GEP-NETs), on the SSR expression as measured by [18F]SiTATE-PET/CT [4]. The study aimed to evaluate the necessity of pausing SSA treatment before [18F]SiTATE-PET/CT.
In total, 77 patients with well-differentiated metastatic or non-resectable GEP-NETs were included in the study. Forty had received SSAs within the last 28 days before PET/CT, whereas 37 patients received no pre-treatment with SSAs. Patients were infused with [18F]SiTATE around 90 mins before PET/CT, with a scan time of 15-20 mins. Maximum and mean standardized uptake value ratios (SUVR) between primary tumors or metastases were compared to background tissue and specific organs in patients with and without SSA pre-treatment.
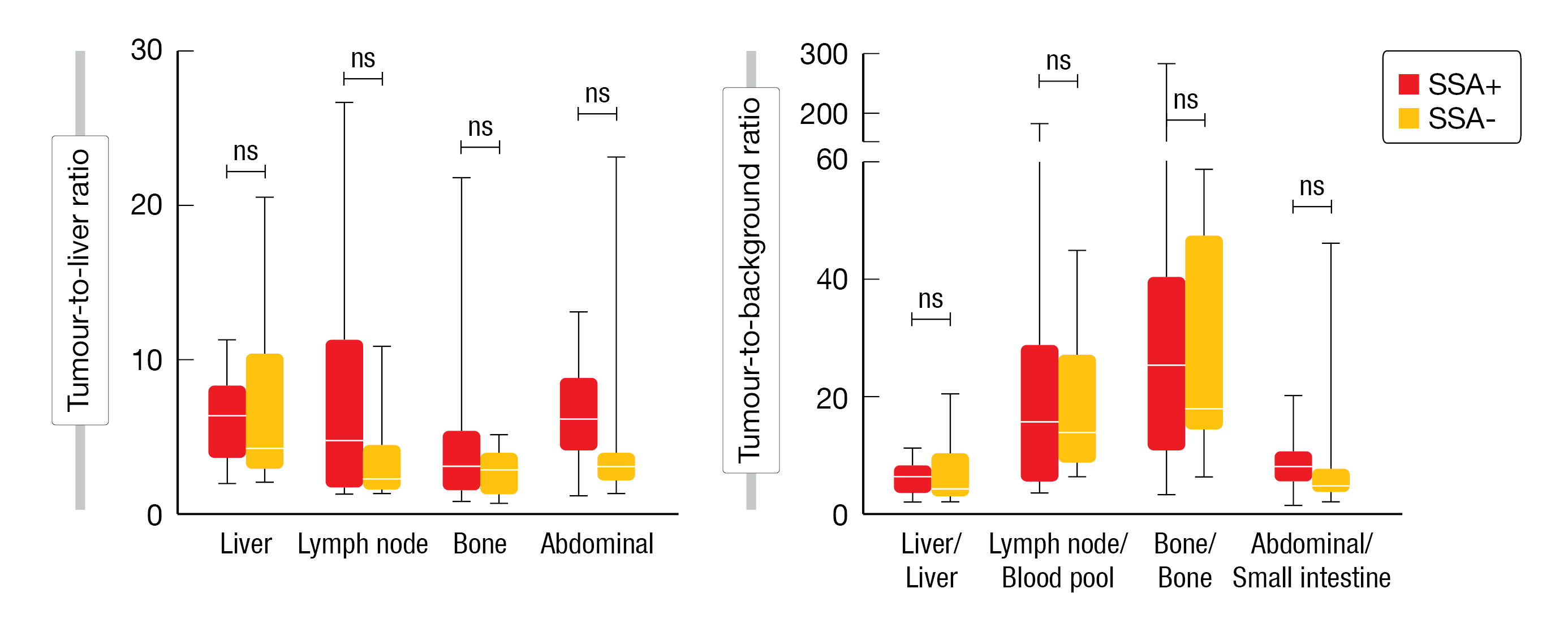
Significantly lower [18F]SiTATE uptake was observed in the spleen and liver of patients who had received prior SSAs, whereas blood [18F]SiTATE was significantly higher compared to SSA treatment-naïve patients. The tumor-to-liver and the specific tumor-to-background SUVRs were not significantly impacted, irrespective of whether the patients had previously received SSAs (Figure 1).
Overall, the data do not support the need for pausing SSA treatment prior to [18F]SiTATE PET/CT imaging in NET patients.
Figure 1: Box-plots representing the tumor-to-liver ratio (A) and tumor-to-background ratio (B) in GEP-NET patients based on whether they previously received SSA for treatment.
SSR-PET/CT could be used as an alternative to liver MRI in NET metastases detection
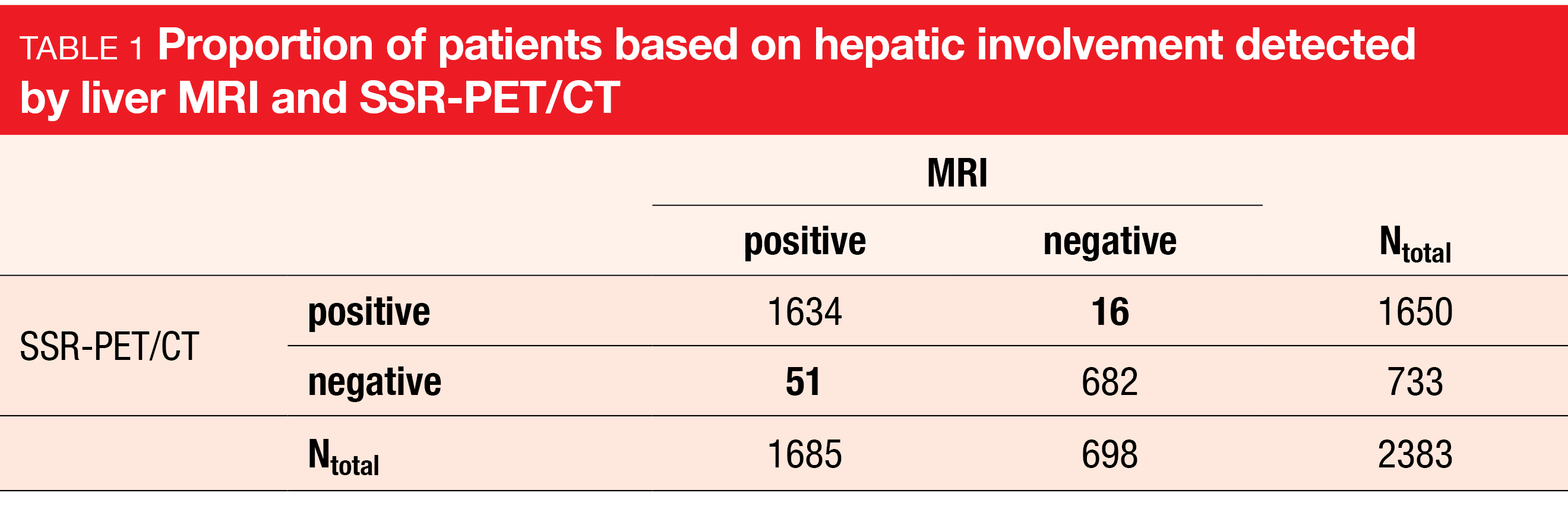
There are no established guidelines on whether to choose SSR-targeting PET/CT or liver magnetic resonance imaging (MRI) for detecting NET liver metastases [5]. Grawe et al. presented data from a retrospective analysis of medical reports of 1000 NET patients (Grade 1 or 2) with SSR-PET/CT and matching liver MRI performed within an interval of 3 months [6]. Hepatic involvement detected by liver MRI but not in SSR-PET/CT were rated false-negative, whereas those observed in SSR-PET/CT but not by MRI were rated false-positive.
Of the 2383 imaging cases included, patient-based metastatic hepatic involvement was reported in 71 % of liver MRI and 69 % of SSR-PET/CT cases (Table 1). There were 2 % false-negative and 1 % false-positive cases. SSR-PET/CT demonstrated a sensitivity of 97.0 % (95 % CI 96.0 %-97.7 %) and a specificity of 97.7 % (95 % CI 96.3 %-98.7 %).
The most frequent reason for false-negative results in SSR-PET/CT was the small size of lesions (<1.2 cm) located in the subcapsular region or near big vessels. The reasons for false-positive findings in SSR-PET/CT were hemangioma, liver cysts, or vessel/bile duct associated with increased tracer uptake.
Overall, the study confirmed the high sensitivity and specificity of SSR-PET/CT in detecting hepatic involvement in NET patients compared to liver MRI imaging as a reference standard. Although SSR-PET/CT could reduce unnecessary biopsies and optimize clinical patient management, awareness of possible pitfalls is essential.
[18F]AIF-NOTA-octreotide shows non-inferiority to [68Ga]Ga-DOTA-SSA PET imaging in NET patients
Gallium-68-labeled SSAs, such as [68Ga]Ga-DOTATATE and [68Ga]Ga-DOTATOC, used in combination with PET/CT imaging, are the gold standard for staging and post-therapy follow-up of SSR-positive NETs [7]. Despite the high tumor detection rates, recent challenges have emerged with using gallium-68 tracers, such as limited availability, high cost, and relatively low throughput. Hence, novel alternatives have emerged, such as fluorine-18 labeled SSR ligands offering advantages concerning availability, production capacity, and image resolution. [18F]AIF-NOTA-octreotide ([18F]AIF-OC) is a promising fluorine-18-labeled SSA alternative for NETs [8]. Two studies were presented at EANM 2022 comparing [68Ga]Ga-DOTATATE versus [18F]AIF-OC PET/CT imaging in NET patients.
Haeger et al. presented data from a prospective trial of stage IV, biopsy-proven NET patients (n=20) who underwent both imaging scans [9]. The maximum and mean SUV were assessed in NET lesions and specific organs on [18F]AIF-OC and [68Ga]Ga-DOTATATE PET/CT images. Tumor-to-liver (TLR) and tumor-to-spleen ratios (TSR) were calculated and compared between the two scans.
[68Ga]Ga-DOTATATE PET was performed before [18F]AIF-OC PET with a mean interval of 12.8 ± 7.9 days (range: 2-30 days) between the scans. The uptake of [68Ga]Ga-DOTATATE was significantly higher than that of [18F]AIF-OC in most organs. However, no statistical differences were observed regarding the comparative analysis of TLR and TSR between both tracers, suggesting a better target-to-background ratio of [18F]AIF-OC than [68Ga]Ga-DOTATATE (Table 2). Few lesions were detected in only one of two scans (three only by [68Ga]Ga-DOTATATE and one only by [18F]AIF-OC), but the differences were not significant.
In another prospective, multicenter trial, [18F]AIF-OC was tested for non-inferiority to 68Ga-DOTA-SSA as a PET imaging tracer in NET patients [10]. Patients with histologically confirmed NET, having undergone [68Ga]Ga-DOTA-TATE/-NOC PET within the last 3 months as routine clinical scans or scheduled for one, and with at least one known tumor lesion outside the head region, were enrolled. Study participants underwent a whole-body PET/CT scan with low-dose CT two hours after IV administration of 4 MBq/kg [18F]AIF-OC. Tumor lesions were counted in consensus by two experienced readers, blinded for patient data and radiopharmaceutical, in random order. Following unblinding, the DR was determined for each scan in every patient as the reference. The differential detection ratio (DDR), the difference in DR between [18F]AIF-OC and [68Ga]Ga-DOTA-TATE/-NOC per patient, was the primary endpoint.
In 75 patients, a total of 4709 unique tumor lesions were counted. Patients had a median interval between the study scan and routine [68Ga]Ga-DOTATATE (n=56) or [68Ga]Ga-DOTANOC (n=19) PET of 7 days (range: -30 to +32 days). The mean DR with [18F]AIF-OC was significantly higher than with [68Ga]Ga-DOTA-TATE/-NOC (91.1 % vs. 75.3 %), with the mean DDR being 15.8 % (95 % CI 9.6 %-22.0 %). The lower margin of its 95 % CI was higher than -15 %, the prespecified boundary for the primary endpoint. The trial was thus positive, demonstrating both non-inferiority and superiority of [18F]AIF-OC compared with [68Ga]Ga-DOTA-TATE/-NOC.
In total, the results from these two studies demonstrated that [18F]AIF-OC is a promising alternative to [68Ga]Ga-DOTATATE in SSR PET imaging for NET patients. More recently published data support [18F]AIF-OC as a valid option in routine clinical practice [11].
REFERENCES
- Bodei L et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40(5):800-816
- Ilhan H et al. Impact of interim PET staging with SSTR-analogs after two cycles of 177Lu-PRRT in NET patients: a multicenter analysis. EANM 2022 (Oral Abstract OP-244)
- Niedermoser S et al. In Vivo Evaluation of 18F-SiFAlin–Modified TATE: A Potential Challenge for 68Ga-DOTATATE, the Clinical Gold Standard for Somatostatin Receptor Imaging with PET. J Nucl Med 2015; 56:1100–1105
- Sheikh GT et al. Comparison of somatostatin receptor expression in patients with neuroendocrine tumours with and without somatostatin analogue treatment imaged with [18F]SiTATE. EANM 2022 (Oral Abstract OP-248)
- Haug AR et al. Neuroendocrine tumor recurrence: diagnosis with 68Ga-DOTATATE PET/CT. Radiology 2014;270(2):517-525.
- Grawe F et al. SSR-PET/CT compared to contrast-enhanced liver MRI in detection of liver metastases in neuroendocrine tumors (NET) – a 15-year retrospective European single-center analysis. EANM 2022 (Oral Abstract OP-252)
- Deroose CM et al. Molecular Imaging of Gastroenteropancreatic Neuroendocrine Tumors: Current Status and Future Directions. J Nucl Med. 2016;57(12):1949-1956.
- Pauwels E, Cleeren F, Tshibangu T, et al. [18F]AlF-NOTA-octreotide PET imaging: biodistribution, dosimetry and first comparison with [68Ga]Ga-DOTATATE in neuroendocrine tumour patients. Eur J Nucl Med Mol Imaging. 2020;47(13):3033-3046.
- Haeger A et al. Comparison of Al[18F]F-NOTA-octreotide and [68Ga]Ga- DOTATATE in Neuroendocrine Tumors. EANM 2022 (Oral Abstract OP-251)
- Pauwels E et al. [18F]AlF-NOTA-octreotide vs. [68Ga]Ga-DOTA-somatostatinanalogue PET in neuroendocrine tumour patients: final results of a prospective multicentre trial. EANM 2022 (Oral Abstract OP-544)
- Pauwels E et al. 18F-AlF-NOTA-octreotide outperforms 68Ga-DOTA-TATE/-NOC PET in neuroendocrine tumor patients: results from a prospective, multicenter study [published online ahead of print, 2022 Oct 20]. J Nucl Med. 2022;jnumed.122.264563
© 2023 Springer-Verlag GmbH, Impressum
More posts
Preface – EANM 2022
Preface – EANM 2022 © private - Oana C. Kulterer, MD, Division of Nuclear Medicine, D


![Table 2 Tumor-to-liver ratio (TLR) and tumor-to-spleen ratio (TSR) of [68Ga]Ga-DOTATATE and [18F]AIF-OC at different metastasis sites](https://memoinoncology.com/wp-content/uploads/2023/02/Picture_EANM2022_3.png)


