Early-stage lung cancer: noteworthy findings for different types of therapy
Phase III data regarding postoperative radiation
Postoperative radiotherapy (PORT) in patients with completely resected NSCLC has been a subject of debate for years. In the absence of robust data confirming the benefit of this intervention, its feasibility was additionally challenged by a multitude of changes that have taken place over the last two decades with respect to patient selection, (neo)adjuvant chemotherapy, surgery, and radiotherapy. Therefore, the large, randomized, phase III LungART study was designed to assess the role of modern mediastinal PORT in patients with completely resected NSCLC and proven N2 nodal involvement. Patients were randomized to either conformal PORT at a dose of 54 Gy delivered over 5.5 weeks (n = 252) or the control arm that went without radiotherapy (n = 249) at centers in France, UK, Germany, Switzerland, and Belgium.
In both arms, 96 % of patients received (neo)adjuvant chemotherapy. Patient selection was predominantly conducted via PET scan. Approximately 40 % had unforeseen N2 disease according to the cTNM classification. According to pTNM or ypTNM, 45 % in each arm showed 1 involved N2 station, and in 52 %, ≥ 2 stations were involved.
In each arm, approximately 80 % of patients underwent lobectomy. The main PORT technique used in the experimental arm was 3D-conformal radiation therapy (89 %). Eleven percent received intensity-modulated radiotherapy. LungART is the first European randomized study to evaluate modern PORT after complete resection in patients selected predominantly with PET who have received (neo)adjuvant chemotherapy. Disease-free survival (DFS) was defined as the primary endpoint.
Increases in cardiopulmonary toxicity
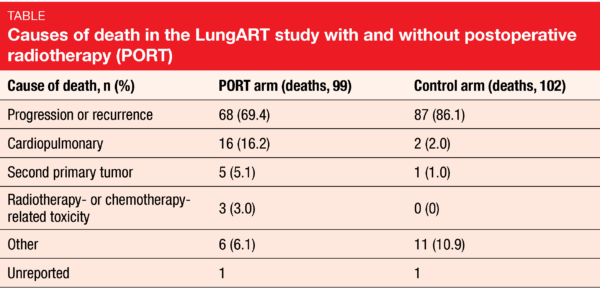
Le Péchoux et al. reported the primary endpoint analysis of LungART at the ESMO 2020 Congress [1]. After a median follow-up of 4.8 years, median DFS was 30.5 vs. 22.8 months in the PORT and control arms, which was equivalent to a non-significant advantage (HR, 0.85; p = 0.16). At 3 years, DSF rates were 47.1 % vs. 43.8 %; these were higher than expected in both arms. A greater proportion of patients in the control arm had mediastinal relapse as first event (46.1 %) compared to those in the PORT arm (25.0 %), although death occurred more frequently as the first event in the PORT-treated patients than in the control patients (14.6 % vs. 5.3 %). OS did not differ across the arms, with 3-year rates of 66.5 % vs. 68.5 %. Overall death rates in the two arms were comparable (39.6 % vs. 41.5 %). However, progression or recurrence was responsible for a greater percentage of fatalities in the control arm, whereas more patients in the PORT arm died due to cardiopulmonary causes (Table).
As expected, early grade 3/4 toxicity occurred more commonly in the experimental arm (11.6 % vs. 7.7 %), as did late grade 3/4 toxicity (14.6 % vs. 8.9 %). Within the first 3 months after randomization, 3 patients in the PORT arm (1.2 %) vs. none in the control arm died due to toxicity (i.e., cardiopulmonary arrest, pneumonitis, infectious pneumonitis). In terms of late grade 5 toxicity, the arms did not differ (1.2 % vs. 0.8 %). Twenty-six patients in the PORT arm (10.8 %) vs. 12 in the control arm (4.9 %) experienced at least one cardiac/pulmonary toxicity grade 3/4 event. Second cancers occurred in 11.1 % vs. 7.2 %; among these patients, second lung cancers were particularly common (39.3 % vs. 22.2 %). This issue clearly requires further analysis with respect to the location of the second tumor, patterns of failure with competing events, and other factors.
Overall, although mediastinal relapse was reduced almost by half in the PORT arm as compared to the control arm, conformal PORT cannot be recommended as a standard of care in all completely resected stage IIIA N2 NSCLC patients due to increased toxicity. Further analyses of the data obtained in the LungART study are planned.
ADAURA: 82 % reduction in CNS recurrence risk
In the double-blind, phase III ADAURA study, adjuvant use of the third-generation EGFR TKI osimertinib induced highly statistically significant and clinically meaningful DFS improvement in patients with completely resected, stage IB-IIIA, EGFR-mutant NSCLC [2]. At the ESMO 2020 Congress, Tsuboi et al. presented a pre-specified exploratory analysis of disease recurrence patterns observed in ADAURA, including CNS [3]. The type of recurrence is a key consideration in resected NSCLC due to worse prognosis in cases of distant events compared to local/regional progression and the significance of the CNS as a common site of distant recurrence in EGFR-mutant disease. Osimertinib has been shown to achieve clinically significant exposure in the brain compared to other EGFR TKIs and has demonstrated greater penetration of the blood-brain barrier [4-6].
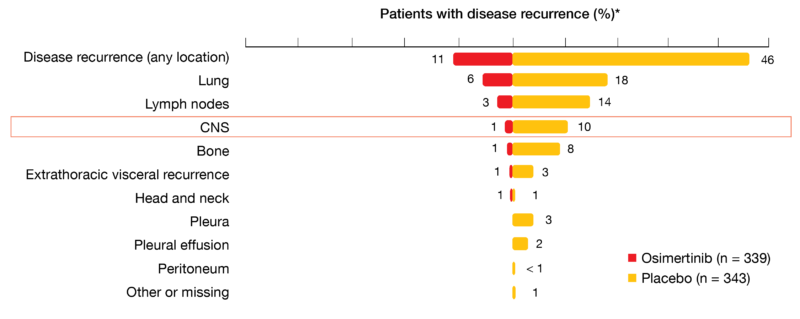
In total, 11 % vs. 46 % of patients treated in the osimertinib and placebo arms of ADAURA, respectively, developed disease recurrence or died. Within the group of relapsing patients in the osimertinib arm, most showed local/regional recurrence (62 %), while 38 % had distant recurrence. In the placebo arm, these proportions were reversed (39 % and 61 % for local/regional and distant recurrence, respectively). Only 1 % of osimertinib-treated patients experienced CNS recurrence, as opposed to 10 % in the control arm (Figure 1). Compared to placebo, the risk of CNS disease recurrence or death observed in the osimertinib arm was reduced by 82 % (HR, 0.18; p < 0.0001). Kaplan-Meier estimates showed a consistently lower cumulative incidence of CNS recurrence in the osimertinib arm. The conditional probability of observing CNS recurrence in the absence of non-CNS recurrence or death at 18 months was < 1 % vs. 9 %. As the authors emphasized in their summary, these results reinforce adjuvant osimertinib as a highly effective, practice-changing treatment for patients with stage IB/II/IIIA, EGFR-mutant NSCLC following complete tumor resection.
Figure 1: ADAURA trial: sites of disease recurrence in the relapsing population
Preoperative atezolizumab: PRINCEPS
In patients with NSCLC, neoadjuvant immune checkpoint inhibition has been shown to induce major pathological response rates in 17 % to 45 % [7, 8]. The phase II PRINCEPS trial tested neoadjuvant atezolizumab monotherapy in patients with stage I to IIIA NSCLC and tumor diameters ≥ 2 cm [9]. Thirty patients unselected for PD-L1 expression received one injection of atezolizumab 1,200 mg, and surgery was performed approximately 4 weeks later, on day 21 to 28. Adjuvant chemotherapy ± radiotherapy according to local standards was possible.
The 2-month tolerance rate (i.e., the rate of patients without major toxicities or morbidities between the start of treatment and 1 month after surgery) constituted the primary endpoint. Major toxicities or morbidities included treatment toxicity leading to a ≥ 15 day delay of surgery, grade ≥ 3 toxicity occurring within 2 months after atezolizumab administration, major postoperative morbidities, any death related to the experimental treatment that occurred from the day of atezolizumab administration to postoperative day 30, and omission of surgery due to early progression.
Besides clinical endpoints, several exploratory objectives were defined that included the adaptive immune response in the microenvironment in post-surgical fresh tissue and blood, the rate of circulating immunomarkers according to liquid biopsy, and molecular profiling at resection, prior to treatment and at disease progression, among others. Negative baseline PD-L1 expression status (< 1 %) was present in 62 % of patients, while 21 % had PD-L1 ≥ 1 % and 17 % were highly PD-L1–positive (≥ 50 %).
No impairment of surgery
Surgery was conducted after a median of 24 days from the administration of atezolizumab. Notably, the resection was not delayed by ≥ 15 days in any patient. Almost all patients underwent lobectomy. In 97 %, R0 resection was possible, while 3 % had R1 resection. Adjuvant radiotherapy was conducted in 29.7 %. Seven of 30 patients (23 %) experienced complications within 1 month after surgery, mostly atrial fibrillation and infection. The complication rate matched the rate that was to be expected in this population. No grade 4/5 complications occurred.
According to RECIST 1.1, 7 % of patients developed partial response. Major pathological responses (i.e., < 10 % residual tumor cells) were observed in 14 %. Pathological response ≥ 50 % (i.e., < 50 % residual cells) was present in 41 %. No patient developed complete pathological response. Analyses demonstrated that pathological responses did not show any correlation with RECIST 1.1 response rates nor metabolic variations according to octreoscan or 18F-FDG PET/CT. However, the researchers found a correlation between pathological response and PD-L1 expression at baseline, as increased PD-L1 levels on tumor cells were associated with more pronounced pathological regression. Quantitative results confirmed this correlation. Also, characterization of the immune infiltration on fresh tissue within 4 hours after resection was initiated. According to flow cytometry analysis, there was a clear correlation between PD-L1 expression and TIGIT expression on lymphocytes.
The authors concluded that one cycle of preoperative atezolizumab was safe and did not impair surgery. Recruitment of 30 patients who do not receive neoadjuvant therapy and who will serve as a control group for translational research is ongoing. The final analysis of the immune contexture in blood and fresh tissue will be performed when the control group will be fully recruited.
Unprecedented OS improvement in PACIFIC at 4 years
The randomized, double-blind, phase III PACIFIC trial has demonstrated benefits of durvalumab as consolidation therapy in patients with stage III, locally advanced, unresectable NSCLC that had not progressed after platinum-based chemoradiotherapy. According to the primary analyses, durvalumab improved both PFS (16.8 vs. 5.6 months; HR, 0.52; p < 0.001) and OS (17.2 vs. 5.6 months; HR, 0.68; p = 0.0025) [10, 11]. At the same time, the PD-L1 inhibitor exhibited a manageable safety profile and did not detrimentally impact patient-reported outcomes [10-12]. These findings established durvalumab after chemoradiotherapy as standard of care in unresectable stage III disease.
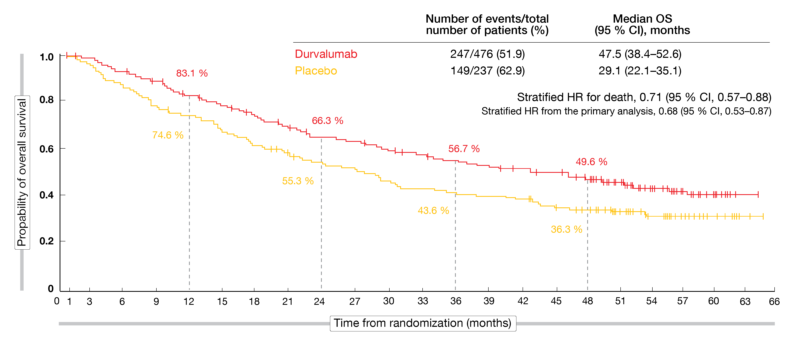
At ESMO 2020, Faivre-Finn et al. reported updated OS and PFS analyses from PACIFIC approximately 4 years after the last patient had been randomized [13]. The findings included the first estimate of median OS for the durvalumab arm, which was 47.5 months, translating into a 29 % mortality reduction compared to placebo (29.1 months; HR, 0.71; Figure 2). At 48 months, OS rates were 49.6 % vs. 36.3 %. According to the updated PFS analysis, the risk of progression and death was reduced by 45 % (17.2 vs. 5.6 months; HR, 0.55). The estimated 48-month PFS rates were 35.3 % vs. 19.5 %. All of the pre-specified subgroups benefited from the durvalumab treatment in terms of OS and PFS with the exception of patients with EGFR mutations, although the small size of this subgroup and the exploratory nature of the analysis preclude definitive conclusions. The checkpoint inhibitor improved PFS in all PD-L1 subgroups as well as OS in patients with PD-L1-positive tumors, while those without PD-L1 expression did not appear to derive any OS benefit from the treatment. However, limitations need to be taken into consideration here, as this was an unplanned post-hoc analysis that was not powered for efficacy.
Overall, durvalumab consolidation after chemoradiotherapy continued to demonstrate durable PFS and sustained OS benefits at 4 years. Ongoing clinical trials are investigating concurrent immune checkpoint inhibition and chemoradiotherapy regimens and might further transform the treatment landscape for patients with unresectable stage III NSCLC.
Figure 2: Long-term overall survival in the PACIFIC trial with durvalumab consolidation versus placebo
REFERENCES
- Le Péchoux C et al., An international randomized trial comparing post-operative radiotherapy (PORT) to no PORT, in patients with completely resected NSCLC and mediastinal N2 involvement. ESMO 2020, LBA3_PR
- Herbst RS et al., Osimertinib as adjuvant therapy in patients with stage IB–IIIA EGFR mutation positive NSCLC after complete tumor resection: ADAURA. J Clin Oncol 38: 2020 (suppl; abstr LBA5)
- Tsuboi M et al., Osimertinib adjuvant therapy in patients with resected EGFR mutated NSCLC (ADAURA): CNS disease recurrence. ESMO 2020, LBA1
- Colclough N et al., Preclinical comparison of the blood brain barrier permeability of osimertinib (AZD9291) with other irreversible next generation EGFR TKIs. Eur J Cancer 2016; 69: S28
- Ballard P et al., Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016; 22(20): 5130-5140
- Vishwanathan K et al., Osimertinib displays high brain exposure in healthy subjects with intact blood-brain barrier: a microdose positron emission tomography study with 11C-labelled osimertinib. Cancer Res 2018; 78(13 Suppl): CT013
- Forde PM et al., Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018; 378: 1976-1986
- Cascone T et al., Neoadjuvant nivolumab or nivolumab plus ipilimumab for resectable non-small cell lung cancer: Clinical and correlative results from the NEOSTAR study. J Clin Oncol 37, 2019 (suppl; abstr 8504)
- Besse B et al., Neoadjuvant atezolizumab for resectable non-small cell lung cancer: results from the phase II PRINCEPS trial. ESMO 2020, 12150
- Antonia SJ et al., Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377(20): 1919-1929
- Antonia SJ et al., Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379(24): 2342-2350
- Hui R et al., Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol 2019; 20(12): 1670-1680
- Faivre-Finn C et al., Durvalumab after chemoradiotherapy in stage III NSCLC: 4-year survival update from the phase PACIFIC trial. ESMO 2020, LBA49
© 2020 Springer-Verlag GmbH, Impressum