News on targeted agents in the advanced setting
Afatinib in squamous-cell carcinoma: update of LUX-Lung 8
Squamous-cell carcinoma of the lung represents approximately 30 % of non–small-cell lung cancer (NSCLC) cases. Until 2015, docetaxel and erlotinib were the only approved second-line treatment options in these patients. Typically, squamous-cell carcinoma of the lung has a high burden of somatic mutations and genomic alterations. Overexpression and dysregulation of EGFR, FGFR1, PI3K and their downstream pathways are implicated in the pathogenesis, providing a rationale for the use of ErbB inhibitors in this setting of major medical need.
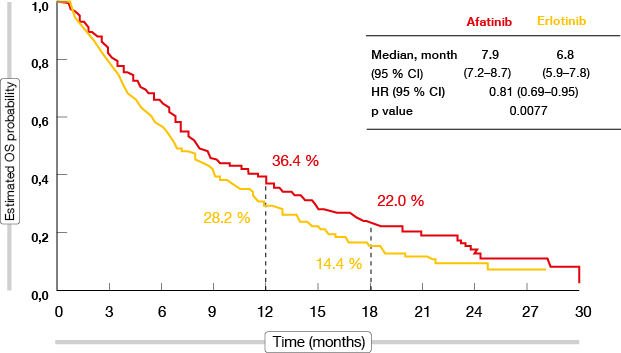
The global, open-label, randomised, phase III LUX-Lung 8 trial compared the irreversible ErbB family blocker afatinib with the reversible EGFR tyrosine kinase inhibitor (TKI) erlotinib in a total of 795 patients with squamous-cell carcinoma of the lung after failure of first-line platinum-based chemotherapy. Compared to erlotinib, afatinib significantly improved progression-free survival (PFS; median 2.4 vs. 1.9 months; HR, 0.82, 95 % CI 0.68-1.00, p = 0.0427) and overall survival (OS; median 7.9 vs. 6.8 months; HR, 0.81, 95 % CI 0.69-0.95; p = 0.0077; Figure 1) [1]. The OS effect of afatinib was consistent across subgroups.
At the ECC, the updated PFS results and exploratory genetic analyses using next-generation sequencing of select tumour samples were reported [2].
Figure 1: Overall survival with afatinib versus erlotinib in LUX-Lung 8
The PFS results significantly favoured afatinib (2.6 vs. 1.9 months; HR, 0.81; p = 0.0103). Furthermore, there was a significant improvement in disease control rate (DCR; 50.5 % vs. 39.5 %; p = 0.002). More patients in the afatinib group had an objective response (5.5 % vs. 2.8 %), and median duration of response was longer than in the erlotinib arm (7.3 vs. 3.7 months). Adverse events (AEs) occurred in both arms at similar rates, which also applied to grade ≥ 3 AEs.
No biomarkers for the selection of patients for treatment with afatinib were identified. According to the biomarker analyses, the prevalence of EGFR genomic aberrations was consistent with prior reports in patients with squamous-cell carcinoma, and no predictive associations between genetic alterations and OS or PFS were observed. Assessment of EGFR immunohistochemistry and blood-based markers is ongoing, as well as further bioinformatics analysis of next-generation sequencing
Quality of life and other outcomes
The outcome improvements obtained in LUX-Lung 8 were accompanied by similar changes in patient-reported outcomes [3]. Prespecified analyses using the European Organisation for Research and Treatment of Cancer (EORTC) core quality-of-life questionnaire (QLQ-C30) and its lung-cancer-specific module (QLQ-LC13) showed significantly higher proportions of patients reporting improved global health status/ quality of life and cough with afatinib than with erlotinib. For dyspnoea and pain, a non-significant advantage of afatinib compared with erlotinib was observed. Afatinib significantly delayed time to deterioration (TTD) of dyspnoea compared to erlotinib, and there was a trend towards delayed TTD of cough. Changes in mean scores over time significantly favoured afatinib over erlotinib for cough (p = 0.0091), dyspnoea (p = 0.0024), and pain (p = 0.0384).
A diarrhoea substudy (n = 63) analysed the time course and severity of diarrhoea using patient diaries at selected centres. In this substudy, the overall incidence of all-grade diarrhoea was similar to that reported in the overall trial population (86.1 % with afatinib, 51. 8 % with erlotinib). Nineteen percent (7 out of 36) of afatinib-treated patients reported grade ≥ 3 diarrhoea, with a mean duration of 3 days. No patient discontinued study treatment due to this AE.
Overall, these analyses confirm the clinical relevance of the improvements observed for PFS, OS and tumour response with afatinib in LUX-Lung 8. The researchers concluded that afatinib should be considered the TKI of choice for second-line treatment of squamous-cell carcinoma of the lung.
Assessment of nintedanib in squamous-cell carcinoma
Nintedanib is an oral triple angiokinase inhibitor that targets factors of three major proangiogenic pathways. Based on the results of the randomised, placebo-controlled, phase III LUME-Lung 1 study [4], nintedanib has been approved in combination with docetaxel in the European Union and in Russia for the treatment of patients with locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma histology after first-line chemotherapy. Investigations in other histological subgroups of NSCLC patients are ongoing. As an example, a multicentre, phase I, dose-escalating study analysing nintedanib in combination with standard doses of gemcitabine and cisplatin for up to 6 cycles shows promising activity for the first-line treatment of patients with advanced squamous-cell carcinoma [5]. Disease control was achieved in 81.3 %, and the 6-month OS rate was 69 %.
Continuous treatment with nintedanib 200 mg twice daily together with cisplatin/ gemcitabine had a manageable safety profile. Nausea, asthenia, decreased appetite, and constipation were the most frequent AEs. The pharmacokinetic profile of nintedanib and its main metabolites in combination with chemotherapy were comparable to previous nintedanib monotherapy trials. There were no relevant interactions between gemcitabine/ cisplatin and nintedanib at the treatment schedule used. Further research is warranted to determine whether antiangiogenic therapy is an effective treatment option in patients with squamous-cell NSCLC.
Reductions in tumour burden in LUME-Lung 1
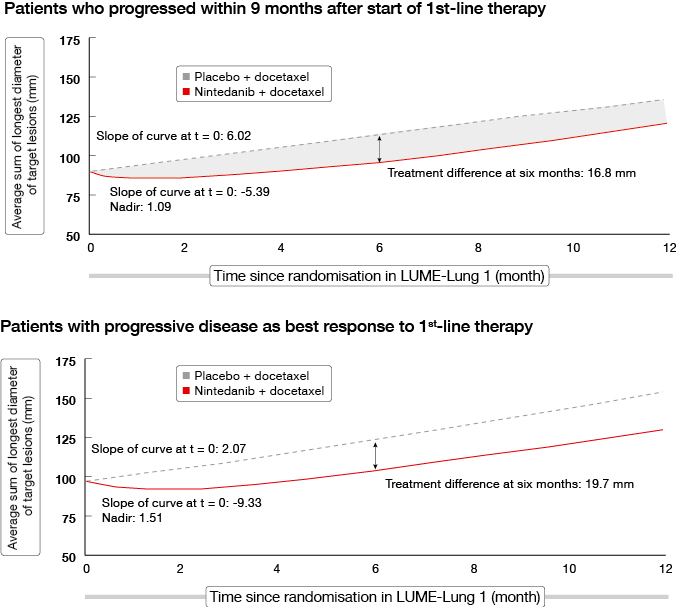
An analysis of the LUME-Lung 1 study investigated the impact of treatment with nintedanib plus docetaxel on tumour growth over time [6]. Tumour burden has been shown to be associated with clinical outcomes in NSCLC; decreases in tumour burden and slowing of tumour growth is an important outcome for patients. Patients with poor prognosis in LUME-Lung 1 were included in this analysis, as well as patients with adenocarcinoma who had progressed within 9 months after start of first-line therapy, patients who had progressive disease as best response to first-line therapy, and all of the patients with squamous-cell carcinoma histology.
Baseline tumour burden was greatest in adenocarcinoma patients with progressive disease as best response to first-line therapy, followed by patients who progressed within 9 months of starting first-line therapy. The combination of nintedanib and docetaxel significantly decreased tumour burden and decelerated tumour growth over time compared to placebo plus docetaxel in all patients with adenocarcinoma histology. Improvements in tumour burden were greatest in those with larger baseline tumour burden. The two groups of patients with the poorest prognosis, as mentioned above, showed similar results (Figure 2).
Figure 2: Tumour growth over time in patients with poor prognosis in LUME-Lung 1
T790M resistance mutation: erlotinib plus bevacizumab
Activating EGFR mutations are found in approximately 15 % of all NSCLC tumours, which provides the basis for EGFR TKI therapy, either as a first-line or later-line treatment option after chemotherapy. However, approximately 60 % of patients who receive EGFR TKI treatment develop the acquired resistance mutation T790M. Identifying strategies to overcome this therapeutic obstacle has become an important area of research.
The open-label, multicentre, phase II ETOP 2-11 BELIEF trial investigated the combination of erlotinib and bevacizumab in patients with advanced non-squamous NSCLC with activating EGFR mutations with and without T790M [7]. The rationale for this trial was the hypothesis that combined VEGFR/ EGFR pathway blockade might be beneficial in the presence of T790M. Patients with activating EGFR mutations were treated with erlotinib 150 mg and bevacizumab 15 mg/kg every 3 weeks until progression. Pre-treatment T790M mutations were identified centrally. Overall, 109 patients were enrolled. Substudy 1 included patients with T790M (n = 37), whereas those without T790M were assessed in substudy 2 (n = 72).
The combination of erlotinib and bevacizumab resulted in an overall 1-year PFS rate of 56.7 % and median PFS of 13.8 months. In patients with documented T790M mutation, the 1-year PFS rate was 72.4 % and the median PFS was 16.0 months; thus, the predefined endpoint for success was reached. Patients without T790M had a 1-year PFS rate of 49.4 % and median PFS of 10.5 months. Those with T790M at baseline fared better across subgroups. With one exception, all patients experienced tumour shrinkage. No unexpected toxicities were identified.
Further investigations using multiple orthogonal methods including digital polymerase chain reaction and multiplex next-generation sequencing are ongoing.
Rociletinib in T790M-negative patients
There is an unmet medical need for new therapies that are active in both T790M-positive and T790M wild-type NSCLC patients who progressed after EGFR TKI treatment. Rociletinib, a novel, oral, selective covalent EGFR TKI, was developed to address key activating mutations together with the T790M mutation.
TIGER-X was a phase I/II study that investigated rociletinib in previously treated, EGFR-mutant patients with advanced or recurrent NSCLC and both T790M-positive (n = 111) and T790M-negative (n = 482) mutation status. Phase 1 of the trial was conducted for dose-escalation, while in phase 2, expansion cohorts were treated. One cohort consisted of second-line patients with disease progression upon one immediate prior TKI, and the other comprised patients beyond second line who had experienced progression upon at least two TKIs or chemotherapy. Treatment was conducted with rociletinib 500 mg, 625 mg or 750 mg, twice daily (BID).
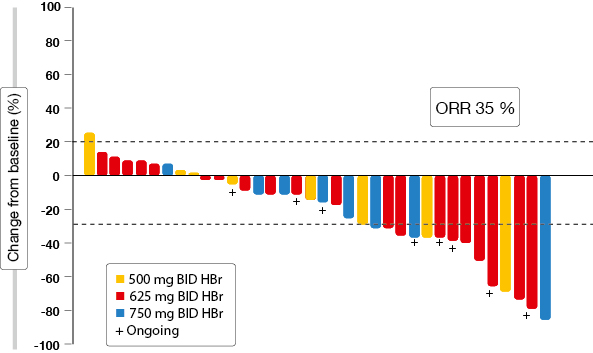
The analysis presented at the ECC focussed on updated results in centrally confirmed tissue T790M-negative patients [8]. Overall response rate (ORR) was 35 % in this population (Figure 3). Disease control occurred in 65 %. A comparison of available tissue-based testing assays (Therascreen® and Cobas®) yielded highly concordant results. Plasma T790M testing revealed an ORR of 45 % and a DCR of 83 % in patients with negative mutation status. The investigators stated that plasma tests may be more representative, especially with extra-thoracic spread. However, the greater rate of false negatives has to be taken into account. The most common treatment-related AEs were similar to those observed in the general TIGER-X patient population.
It is possible that the efficacy of rociletinib in T790M-negative patients is driven in part by clonal heterogeneity, as not all cells express T790M, or by the inhibitory effect on insulin-like growth factor 1 receptor (IGF-1R) and insulin receptor (IR) kinases. IGF-1R/ IR might drive resistance to initial EGFR inhibitor therapy. TKI retreatment may not be a likely explanation, as the majority of patients had a recent history of progression on EGFR TKI therapy. In view of these results, the clinical profile of rociletinib in T790M-negative NSCLC patients continues to be encouraging. The efficacy of rociletinib in this population is currently being evaluated in the TIGER-2 and TIGER-3 clinical studies.
Activity of rociletinib against the background of CNS disease
Central nervous system (CNS) metastases occur in up to 50 % of advanced NSCLC cases. In EGFR-mutant patients treated with first-generation EGFR TKIs, the 2-year CNS progression rate is 21 %, and 40 % among those with a prior history of CNS disease.
Patients with asymptomatic treated CNS metastases were allowed to participate in the TIGER-X study. A T790M-positive biopsy was required at the time of study entry in phase 2 of the trial. In this phase, a total of 41 % of patients with a history of CNS disease were treated. This factor did not appear to affect response rates, as the ORRs among patients without and with a history of CNS metastases were 58 % and 45 %, respectively [9]. DCRs were 92 % and 75 %, respectively. Also, the CNS radiation rate on study was assessed on the assumption that CNS radiation and post-progression TKI therapy can be used as a surrogate for CNS progression on rociletinib. Based on these parameters, it was estimated that 15 % of progressing patients might have CNS progression on rociletinib, which is lower than available historical data on erlotinib. Rociletinib might provide ongoing extracranial disease control in patients who received CNS radiation due to progressive disease.
The study treatment was generally well tolerated, with 2.5 % of patients discontinuing the trial due to treatment-related AEs. Hyperglycaemia and diarrhoea were the most frequent toxicities. Hyperglycaemia was the only grade ≥ 3 AE observed in >10 % of patients; it occurred in 17 %. In the 500 mg BID group, only 9.6 % of patients without a history of diabetes or glucose impairment had post-baseline glucose measurements that exceeded 250 mg/dL at least twice. Interstitial lung disease did not occur. Additional data on the clinical efficacy of rociletinib in patients without and with a history of CNS metastases continue to be generated in TIGER-X and the other TIGER studies.
Figure 3: Waterfall plot of best RECIST response for target lesions obtained with rociletinib in T790M-negative patients
REFERENCES
- Soria JC et al., Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015; 16(8): 897-907
- Goss GD et al., Phase III trial of afatinib versus erlotinib in patients with squamous cell carcinoma of the lung (LUX-Lung 8): EGFR molecular aberrations and survival outcomes. ECC 2015, abstract 3084
- Popat S et al., Second-line afatinib versus erlotinib in patients with advanced squamous cell carcinoma of the lung: patient-reported outcome data from the global LUX-Lung 8 phase II trial. ECC 2015, abstract 3085
- Reck M et al., Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014; 15: 143-155
- Forster M et al., Nintedanib in combination with cisplatin/gemcitabine as 1st-line therapy for advanced squamous non-small cell lung cancer. ECC 2015, abstract 3112
- Reck M et al., Tumour growth over time in patients with advanced non-small cell lung cancer treated with nintedanib + docetaxel or placebo + docetaxel: analysis of data from the LUME-Lung 1 study. ECC 2015, abstract 3102
- Stahel R et al., A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced non-small-cell lung cancer (NSCLC) with activating epidermale growth factor receptor (EGFR) mutations with and without T790M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial. ECC 2015, abstract 3BA
- Solomon B et al., Rociletinib treatment and outcomes in non-small cell lung cancer (NSCLC) patients with negative central testing for T790M. ECC 2015, abstract 3104
- Varga A et al., Activity of rociletinib in EGFR mutant NSCLC patients with a history of CNS involvement. ECC 2015, abstract 3009