Pivotal results and sub-analyses in the field of immunotherapy
CheckMate 017: favourable quality-of-life outcomes
Binding of the inhibitory receptor PD-1 to its ligands, PD-L1 and PD-L2, inhibits T-cell responses. This pathway can be exploited by tumours to escape T-cell-induced anti-tumour activity. Therefore, it is a target for antibodies designed to block this mechanism, with the aim of enhancing immune responses. The fully human anti-PD-1 antibody nivolumab has already been approved in the US and Europe for use in pre-treated patients with advanced squamous NSCLC. In the phase III CheckMate 017 study, nivolumab demonstrated superior OS compared with docetaxel in this population (9.2 vs. 6.0 months; HR, 0.59; p = 0.00025) [1]. This also applied to PFS (3.5 vs. 2.8 months; p = 0.0004).
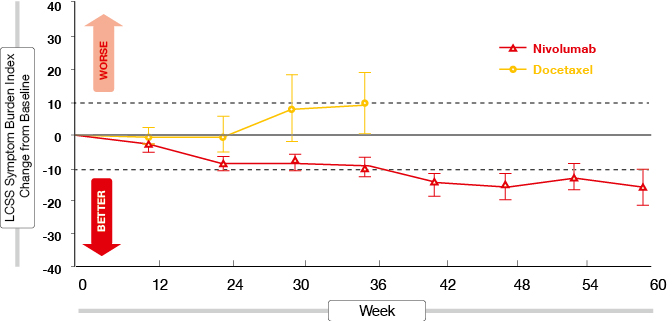
One of the predefined secondary endpoints of CheckMate 017 was improvement of symptoms. The results of this analysis were presented at the ECC, together with those of an exploratory quality-of-life analysis that included patient-reported outcomes using the EuroQoL-5 Dimensions (EQ-5D) Utility Index and the EQ-5D visual analogue scale [2]. Nivolumab was superior to docetaxel according to both scales. On treatment, changes for nivolumab indicated stable or improved health status, while changes for docetaxel suggested stable or declining health status. In patients treated with docetaxel, their health status deteriorated at a significantly faster rate than for the patients on nivolumab. Symptom burden according to the Lung Cancer Symptom Scale was stable from baseline in patients remaining on treatment with docetaxel, but improved meaningfully in those remaining on nivolumab (Figure 1).
Figure 1: Symptom burden on treatment with nivolumab versus docetaxel
Eighteen-month update of CheckMate 057
For patients with advanced non-squamous NSCLC, the phase III, randomised CheckMate 057 trial also demonstrated the superiority of nivolumab over docetaxel. In this pre-treated cohort, the immunotherapeutic agent conveyed benefits with regard to OS (12.2 vs. 9.4 months; HR, 0.73; p = 0.0015) and ORR (19 % vs. 12 %; p = 0.0246) [3]. OS rates at 1 year were 51 % and 39 %, respectively. The biomarker analysis showed a correlation between PD-L1 expression and OS, as well as PFS.
The 18-month update continued to favour nivolumab treatment, with OS rates of 39 % versus 23 % [4]. This difference translated into a 28 % reduction in mortality risk (HR, 0.72), which was highly significant (p = 0.0009). According to subgroup analysis, the ORRs were superior for nivolumab in all subsets, with the exception of never smokers and EGFR-positive patients. As in the primary analysis, nivolumab demonstrated clinical benefit in patients expressing PD-L1. Depending on the degree of expression, the median OS ranged from 17.7 months to 19.9 months with nivolumab (vs. 8.0 to 9.0 months with docetaxel), whereas in non-expressors, survival outcomes did not differ between the two treatment arms. Response rates also favoured nivolumab (31 % to 37 %) over docetaxel (12 % to 13 %) in the patients expressing PD-L1. In PD-L1–negative patients, on the other hand, the ORR was slightly higher with docetaxel than nivolumab. The median duration of response was longer for nivolumab in both expressors (16.0 vs. 5.6 months) and non-expressors (18.3 vs. 5.6 months).
Patient-reported outcomes were assessed according to the Average Symptom Burden Index. By week 12, symptom improvement rates were similar for nivolumab and docetaxel, at 17.8 % and 19.7 %, respectively. Lung Cancer Symptom Scale scores remained stable throughout treatment, in both arms.
Rapid tumour reduction with pembrolizumab: KEYNOTE-001
Two doses of the humanised anti-PD-1 antibody pembrolizumab were tested in the KEYNOTE-001 trial in 449 previously treated patients who suffered from advanced NSCLC of any histology [5]. The analysis showed similar efficacy and safety with pembrolizumab 2 mg/kg and 10 mg/kg, supporting 2 mg/kg every three weeks as an effective dose in NSCLC. The correlation of efficacy with PD-L1 expression was assessed on the basis of tumour samples that were scored according to the percentage of tumour cells with membranous PD-L1 staining (tumour proportion score; TPS).
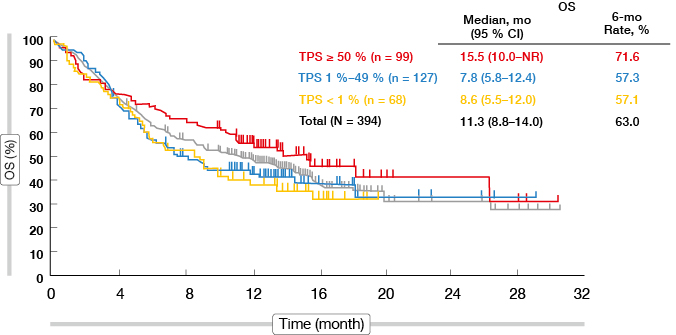
Indeed, tumour shrinkage was more pronounced in patients showing PD-L1 TPS ≥ 50 % compared to those with PD-L1 TPS < 50 % (74.2 % vs. 51.7 %). ORR was highest in the PD-L1 TPS ≥ 50 % cohort in both dose groups. While tumour size at baseline did not affect ORR, responses were observed less frequently in patients with liver metastases (13.6 %) than in those without (21.2 %). Rapid tumour reductions occurred predominantly in the cohort with TPS ≥ 50 %; also, the duration of response was longest in this subgroup. Correspondingly, patients with TPS ≥ 50 % benefited most with regard to OS, as they experienced median survival of 15.5 months in the 10-mg dose group (Figure 2). The same correlation applied to PFS: at 6 months, 49.9 % of those with TPS ≥ 50 % were progression-free, compared to 25.3 % and 23.2 % in the groups with TPS 1 % to 49 % and < 1 %, respectively. Overall, PD-L1 TPS ≥ 50 % was identified as a marker for patients with the greatest likelihood to derive benefit from pembrolizumab treatment. Randomised data will be generated in the ongoing KEYNOTE-010 study that is evaluating pembrolizumab 2 mg/kg or 10 mg/kg three-weekly compared to docetaxel.
Figure 2: Overall survival according to tumour proportion score in the pembrolizumab 10 mg cohort of KEYNOTE-001
Is PD-L2 expression important?
A laboratory analysis addressed the potential relevance of PD-L2, one of the two known binding partners of PD-1, with respect to the efficacy of anti-PD-1 therapies in various cancers, such as pembrolizumab [6]. PD-L2 negatively regulates T cells in immune responses. Some tumours show documented PD-L2 expression by tumour cells and/or infiltrating immune cells. PD-L2 has a role in mediating the severity of disease in murine models of autoimmunity, hypersensitivity and infection.
Archival samples obtained from pembrolizumab-treated patients with a range of tumours, among them NSCLC, were evaluated. PD-L2 expression was found in various degrees on tumour cells, stromal cells, and endothelium. In NSCLC, stromal cell expression outweighed both tumour cell and endothelial expression. PD-L2 and PD-L1 expression were generally concurrent, although discordance was observed in both directions. Notably, some NSCLC samples showed PD-L1 expression in the absence of PD-L2. However, as for the other tumour types, the agreement was highly significant. A pilot analysis in patients with squamous-cell carcinoma of the head and neck suggests that PD-L2 status predicts the outcome on pembrolizumab treatment after adjusting for the impact of PD-L1 expression.
Primary analysis of POPLAR
By inhibiting PD-L1 instead of PD-1, additional benefits can be gained, because this approach leaves the PD-L2/ PD-1 interaction intact, thus potentially preserving peripheral homeostasis. The humanised anti-PD-L1 antibody atezolizumab works through inhibition of the binding of PD-L1 to PD-1 and B7.1. This mechanism can restore anti-tumour T-cell activity and enhance T-cell priming. The interim analysis of the randomised phase II POPLAR study demonstrated promising ORR with atezolizumab monotherapy in the second-line and third-line treatment of metastatic or locally advanced NSCLC, as compared to docetaxel [7]. This effect correlated with PD-L1 expression on tumour cells (TC) and/or tumour-infiltrating immune cells (IC). Expression was defined according to four cut-off levels (0 to 3 for both TC and IC), and four cohorts were investigated (TC0 and IC0; TC1/2/3 and IC1/2/3; TC2/3 and IC2/3; TC3/IC3).
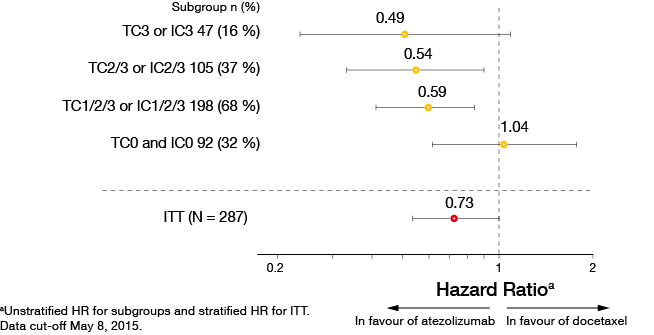
The primary analysis presented at ECC showed significant OS improvements in both squamous and non-squamous NSCLC [8]. Median OS in the entire cohort was 12.6 versus 9.7 months with atezolizumab and docetaxel, respectively (HR, 0.73; p = 0.040). Again, the results were in favour of atezolizumab in patients expressing PD-L1 on TC or IC, with higher expression indicating improved OS (Figure 3). The same correlation applied to PFS and ORR. Both TC and IC expression were shown to be independent predictors of survival improvement with atezolizumab. Duration of response was 14.3 versus 7.2 months with atezolizumab and docetaxel, respectively. The safety profile of atezolizumab was consistent with previous studies and compared favourably to chemotherapy. Several ongoing trials are assessing atezolizumab in various settings.
Figure 3: Overall survival according to PD-L1 expression in POPLAR (atezolizumab vs. docetaxel)
BIRCH: atezolizumab in a PD-L1–enriched cohort
In contrast to POPLAR, the single-arm, phase II BIRCH trial tested atezolizumab in a PD-L1–selected NSCLC population [9]. PD-L1 expression was tested using immunohistochemistry (IHC); only patients with TC2/3 or IC2/3 were included in the study. Three cohorts of patients with locally advanced or metastatic tumours received atezolizumab as first line (Cohort 1) or as subsequent lines (Cohort 2: one prior platinum chemotherapy; Cohort 3: at least two prior chemotherapies, including one platinum-containing regimen). The primary endpoint was ORR, according to the Independent Review Facility.
BIRCH met its primary endpoint in all of the predefined subgroups. ORR was highest in patients with maximum TC or IC expression (TC3, IC3) in all lines. The OS data are not yet mature, but the 6-month OS rates were shown to be consistent with the POPLAR results for the second-line and third-line setting. In the TC2/3 and IC2/3 cohorts, OS was 76 % and 71 % at 6 months for second and third line, respectively. In the TC3 and IC3 cohorts, it was 80 % and 75 %, respectively. The majority of adverse events were grades 1 or 2, and no unexpected safety signals occurred.
REFERENCES
- Spigel DR et al., A phase III study (CheckMate 017) of nivolumab (NIVO; anti-programmed death-1 [PD-1] vs docetaxel (DOC) in previously treated advanced or metastatic squamous (SQ) cell non-small cell lung cancer (NSCLC). J Clin Oncol 33; 2015 (suppl; abstr 8009)
- Reck M et al., Evaluation of overall health status in patients with advanced squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 017. ECC 2015, abstract 3011
- Paz-Ares L et al., Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J Clin Oncol 33; 2015 (suppl; abstr LBA109)
- Horn L et al., Phase 3, randomized trial (CheckMate 057) of nivolumab (NIVO) vs docetaxel (DOC) in advanced non-squamous (non-SQ) non-small cell lung cancer (NSCLC): Subgroup analyses and patient reported outcomes (PROs). ECC 2015, abstract 3010
- Soria J-C et al., Efficacy and Safety of Pembrolizumab (Pembro; MK-3475) for Patients (Pts) With Previously Treated Advanced Non-Small Cell Lung Cancer (NSCLC) Enrolled in KEYNOTE-001. ECC 2015, abstract 33LBA
- Yearley JH et al., PD-L1 expression in human tumors: relevance to anti-PD-1 therapy in cancer. ECC 2015, abstract 18LBA
- Spira A et al., Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR). J Clin Oncol 33, 2015 (suppl; abstr 8010)
- Vansteenkiste J et al., Atezolizumab monotherapy vs docetaxel in 2L/3L non-small cell lung cancer: Primary analyses for efficacy, safety and predictive biomarkers from a randomized phase II study (POPLAR). ECC 2015, abstract 14LBA
- Besse B et al., Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L1-selected non-small cell lung cancer (NSCLC). ECC 2015, abstract 16LBA