Targeted approaches in various B-cell malignancies
Zanubrutinib plus rituximab
BTK inhibitors are active in many B-cell malignancies such as mantle cell lymphoma, CLL and Waldenström’s macroglobulinemia, but also in diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and marginal zone lymphoma (MZL). Zanubrutinib is currently being assessed in pivotal phase II and III studies in all of these indications. An ongoing, single-arm, multicenter, phase II study is evaluating zanubrutinib plus rituximab in patients with relapsed/refractory non-germinal center B-cell-like (non-GCB) DLBCL (n = 20), FL (n = 16) and MZL (n = 5). While zanubrutinib is administered at a dose of 160 mg twice daily until progression, patients are receiving rituximab 375 mg/m2 on days 1, 8, 15 and 22 of cycle 1 and subsequently on day 1 of cycles 4, 6, 8, and 10.
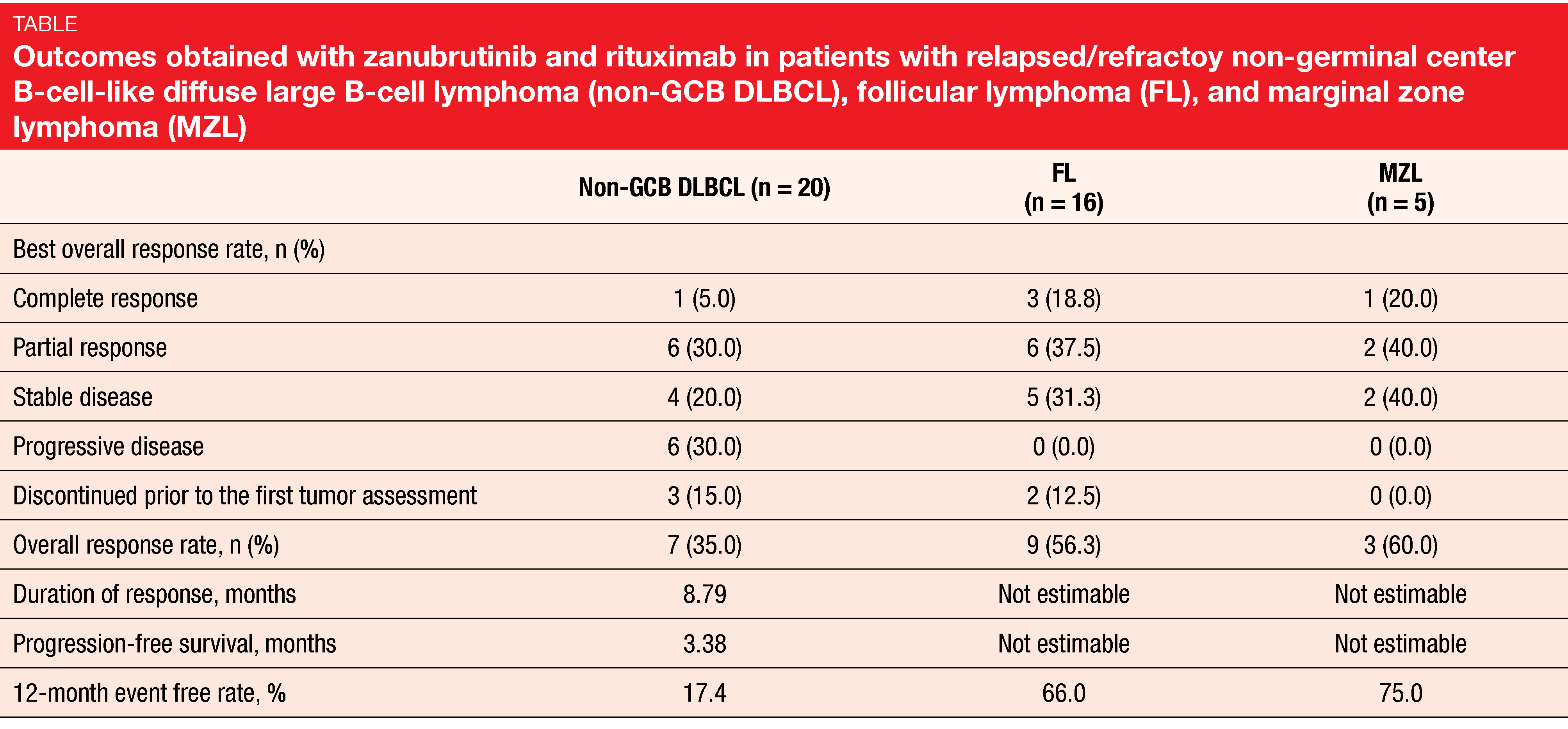
The preliminary results reported by Zhang et al. at the EHA 2020 Congress indicated anti-tumor activity of the combination in all three entities [1]. After a median follow-up of 10.3 months, 34.1 % of patients were still on treatment at data cut-off. Overall response rates were 35.0 %, 56.3 % and 60.0 % for non-GCB DLBCL, FL, and MZL, respectively (Table). In each group, at least one patient achieved complete response. At 12 months, the percentages of event-free patients were 17.4 %, 66.0 % and 75.0 %, respectively. The safety profile observed in this study matched previous results for zanubrutinib. Infections occurred in 34.1 % (grade ≥ 3 events, 9.8 %) and hemorrhage in 26.8 % (no grade ≥ 3 events). As the authors noted, these findings encourage further investigation of the combination of zanubrutinib and anti-CD20 antibodies in FL and MZL and prompted the development of mechanism-based combinations as well as biomarker-driven individualized treatment in patients with non-GCB DLBCL.
Non-GCB DLBCL: biomarker-related outcomes
The non-GCB subtype of DLBCL is associated with poor clinical outcomes [2]. Yang et al. presented data on the activity of zanubrutinib in 121 patients with relapsed/refractory non-GCB DLBCL treated with the BTK inhibitor as monotherapy (n = 79) or in combination with anti-CD20 antibodies (n = 42) in four clinical studies conducted in the phase I and II settings [3]. Also, results of biomarker identification, which has become the focus of DLBCL research, were reported.
Zanubrutinib alone or combined with an anti-CD20 antibody showed activity in the overall non-GCB DLBCL population. The unadjusted ORR was similar across the four trials, with an average of 30 %. Median PFS ranged from 2.8 to 4.9 months, and median OS ranged from 8.4 to 11.8 months. According to the retrospective biomarker analysis, certain subsets of patients derived greater benefit from the treatment. In the group of 56 patients for whom HTG gene expression profiles were established, PAX5 expression was higher in monotherapy responders than in non-responders. Likewise, PIM1, BCL2, and FOXP1 expression was higher in combination therapy responders than in non-responders. In the group with NGS panel data, those with CD79B mutations (n = 25) showed significantly higher ORR than patients without these mutations (n = 52) according to the pooled analysis (60 % vs. 26.9 %; p = 0.005).
Durable responses in marginal zone lymphoma
Compared with chemotherapy-based approaches, BTK inhibitors have shown improved efficacy and tolerability in MZL [4]. Clinical evidence in this field was generated by the first-in-human, open-label, multicenter, phase I/II AU-003 trial assessing the efficacy and safety of single-agent zanubrutinib [5]. AU-003 included a total of 384 patients with B-cell malignancies 20 of whom had relapsed/refractory MZL.
After a median follow-up of 27.1 months, zanubrutinib elicited durable responses in the MZL subgroup. Here, the ORR was 80 %, with 15 % and 65 % of patients obtaining CR and PR, respectively. At 18 months, 66.2 % were still responding to treatment. PFS and OS rates at 24 months amounted to 59.4 % and 83.2 %, respectively. Median PFS had not been reached yet.
Responses emerged in all MZL subtypes; for the extranodal, nodal and splenic types, ORRs were 77.8 %, 100 % and 66.7 %. The zanubrutinib therapy demonstrated a favorable safety profile. One patient discontinued treatment due to an AE. Among AEs of interest, infections occurred most commonly, whereas no patient experienced atrial fibrillation or flutter.
Safety of acalabrutinib in a range of entities
Furman et al. provided an overall summary of the safety profile of acalabrutinib when used as monotherapy in various mature B-cell malignancies [6]. The authors analyzed pooled data from nine clinical studies investigating acalabrutinib in patients with CLL/SLL, prolymphocyctic leukemia, Richter transformation, mantle cell lymphoma, Waldenström’s macroglobulinemia, activated B-cell-like subtype of DLBCL, FL, and multiple myeloma. In these studies, acalabrutinib was administered orally once or twice daily until progression, at total daily doses of 100 mg to 400 mg. Most patients received acalabrutinib 100 mg twice daily. Among the 1,040 included patients, 366 (35 %) were treatment-naïve, while 674 (65 %) had relapsed or refractory disease.
At the time of the analysis, the median follow-up was 26.4 months, and 65 % of patients remained on acalabrutinib treatment at data cut-off. In those who had discontinued treatment, progression was the most common reason. The median relative dose intensity was 98.7 %. Among AEs, headache and diarrhea occurred most frequently and were predominantly grade 1 and 2. Grade ≥ 3 AEs mainly included neutropenia (11.2 %), anemia (7.8 %), and pneumonia (5.1 %). AEs led to dose modifications, dose delays and treatment discontinuation in 4 %, 38 % and 9 %, respectively. Most events of clinical interest such as atrial fibrillation and hypertension were low-grade and infrequent. Twelve percent of patients developed second primary malignancies, primarily non-melanoma skin cancer. Overall, these results support the long-term safety of acalabrutinib in various B-cell malignancies including relapsed/refractory mantle cell lymphoma and CLL.
Polatuzumab/obinutuzumab/venetoclax
Induction treatment with the antibody-drug conjugate polatuzumab in combination with obinutuzumab and venetoclax was tested in the setting of relapsed/refractory FL. At the EHA Congress, Yuen et al. reported a pre-planned interim analysis of this phase Ib/II trial [7]. Complete response at the end of induction (EOI) was defined as the primary efficacy endpoint. The dose escalation and dose expansion populations comprised 33 and 38 individuals, respectively. At the time of the interim analysis, 15 patients had completed induction and constituted the efficacy-evaluable population.
Polatuzumab plus obinutuzumab and venetoclax showed promising activity. Eighty-seven percent of patients responded at EOI, and 60 % of these were complete responders. The triple combination proved tolerable, with the safety profile being consistent with the known profiles of the individual drugs. Infections, diarrhea, nausea, neutropenia and fatigue occurred most commonly. AEs were manageable with supportive care. Treatment discontinuations due to AEs occurred in 14 %, dose delays/interruptions in 54 % and dose reductions in 32 %. Meanwhile, enrollment has been completed for the phase II expansion (n = 40), and the results will be presented at a future meeting.
Findings with the PI3Kδ inhibitor ME-401
Pagel et al. sought to determine the efficacy and safety of the novel oral PI3Kδ inhibitor ME-401 in patients with relapsed/refractory FL and other B-cell malignancies [8]. ME-401 has been developed to fulfill the criteria of optimal pharmaceutical properties, such as long half-life and high potency. An intermittent schedule has been evaluated as a strategy to mitigate delayed immune-related toxicities associated with the continuous daily delivery of oral PI3K inhibitors; here, daily dosing in cycles 1 and 2 is followed by daily dosing for 1 week and treatment interruption for 3 weeks in later cycles. ME-401 was tested using the intermittent schedule in a phase Ib, single-arm, dose-escalation/dose-expansion study. Overall, 57 patients were recruited. Two treatment groups received either ME-401 60 mg daily as monotherapy or 60 mg daily in combination with 4 doses of rituximab 375 mg/m2 weekly followed by 4 doses on day 1 of cycles 3 to 6.
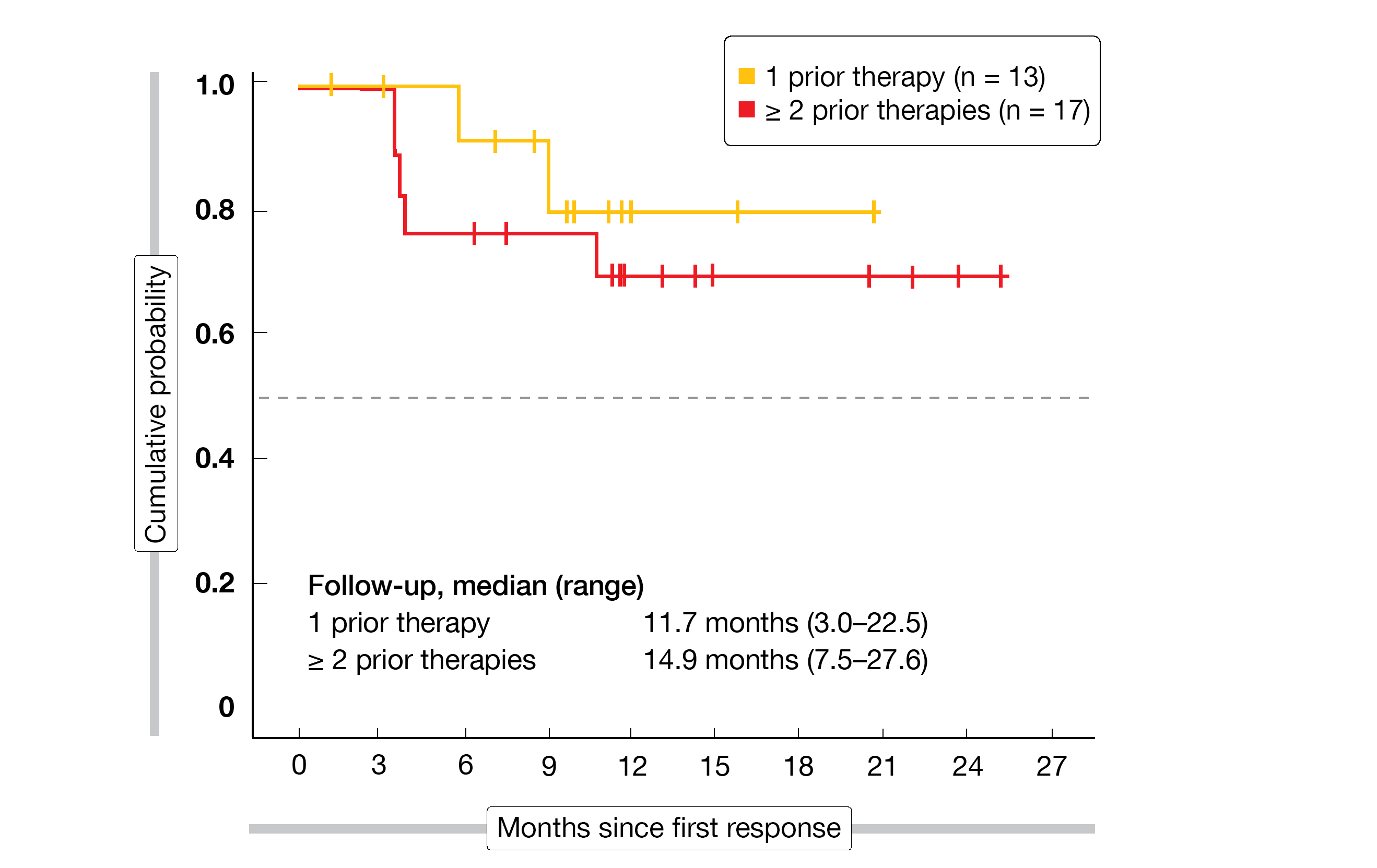
ME-401 gave rise to high ORR in patients with FL (83 %) and CLL/SLL (89 %). CRs were achieved in 22 % and 11 %, respectively. In the FL setting, median duration of response had not been reached after a follow-up of 13.2 months. Durable responses were achieved here irrespective of prior lines of therapy (Figure), treatment group (15.4 vs. 12.8 months with ME-401 monotherapy and ME-401 plus rituximab, respectively), and tumor bulk (12.5 vs. 13.3 months for ≥ 5 cm and < 5 cm, respectively).
Grade ≥ 3 AEs of special interest occurred infrequently and were restricted to the first two cycles. Treatment was discontinued due to AEs in 7 %. The evaluation of ME-401 on the intermittent schedule as a single-agent as well as in combination regimens is ongoing. TIDAL, a global phase II trial assessing ME-401 monotherapy on the intermittent schedule in pretreated FL patients is currently enrolling.
Figure 1: NEOSTAR: pathological response rates obtained with
REFERENCES
- Zhang Q et al., Zanubrutinib in combination with rituximab in patients with relapsed/refractory non-Hodgkin lymphoma. EHA 2020, abstract EP1271
- Alizadeh AA al., Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403(6769): 503-511
- Yang H et al., Biomarker identification in relapsed/refractory non-germinal center B-cell-like diffuse large B-cell lymphoma treated with zanubrutinib. EHA 2020, abstract EP1246
- Noy A et al., Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 2017; 129: 2224-2232
- Tedeschi A et al., Phase 1/2 study of single-agent zanubrutinib in patients with relapsed/refractory marginal zone lymphoma. EHA 2020, abstract EP1165
- Furman RR et al., Safety of acalabrutinib monotherapy in mature B-cell malignancies: pooled analysis from clinical trials. EHA 2020, abstract EP698
- Yuen S et al., Polatuzumab vedotin + obinutuzumab + venetoclax in patients with relapsed/refractory follicular lymphoma: interim analysis of a phase Ib/II trial. EHA 2020, EP1162
- Pagel J et al., The PI3Kδ inhibitor ME-401 is well tolerated on intermittent schedule and produces a high rate of durable responses in relapsed/refractory indolent B-cell malignancies. EHA 2020, abstract EP1174