CLL/SLL: current perspectives across a range of potent agents
ALPINE: zanubrutinib vs. ibrutinib
The introduction of effective inhibitors of B-cell receptor signaling such as the BTK inhibitor ibrutinib has transformed the treatment of patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). The irreversible, potent, next-generation BTK inhibitor zanubrutinib has been designed to maximize BTK occupancy and minimize off-target inhibition of other kinases [1]. Based on the assumption that zanubrutinib might offer advantages over ibrutinib in terms of toxicity and efficacy, the randomized, phase III head-to-head ALPINE study was conducted at 129 centers in 15 countries to compare zanubrutinib 160 mg twice daily (n = 207) with ibrutinib 420 mg/d (n = 208) in patients with relapsed/refractory CLL or SLL after ≥ 1 prior systemic therapy. Approximately 20 % in both arms had deletion 17p and/or mutant TP53, and deletion 11q was present in 29.5 % and 26.4 %, respectively. Overall response rate (ORR, i.e., partial plus complete responses) non-inferiority and superiority as assessed by the investigator constituted the primary endpoint. At EHA 2021, Hillmen et al. presented the pre-planned interim analysis for the first 415 patients [2].
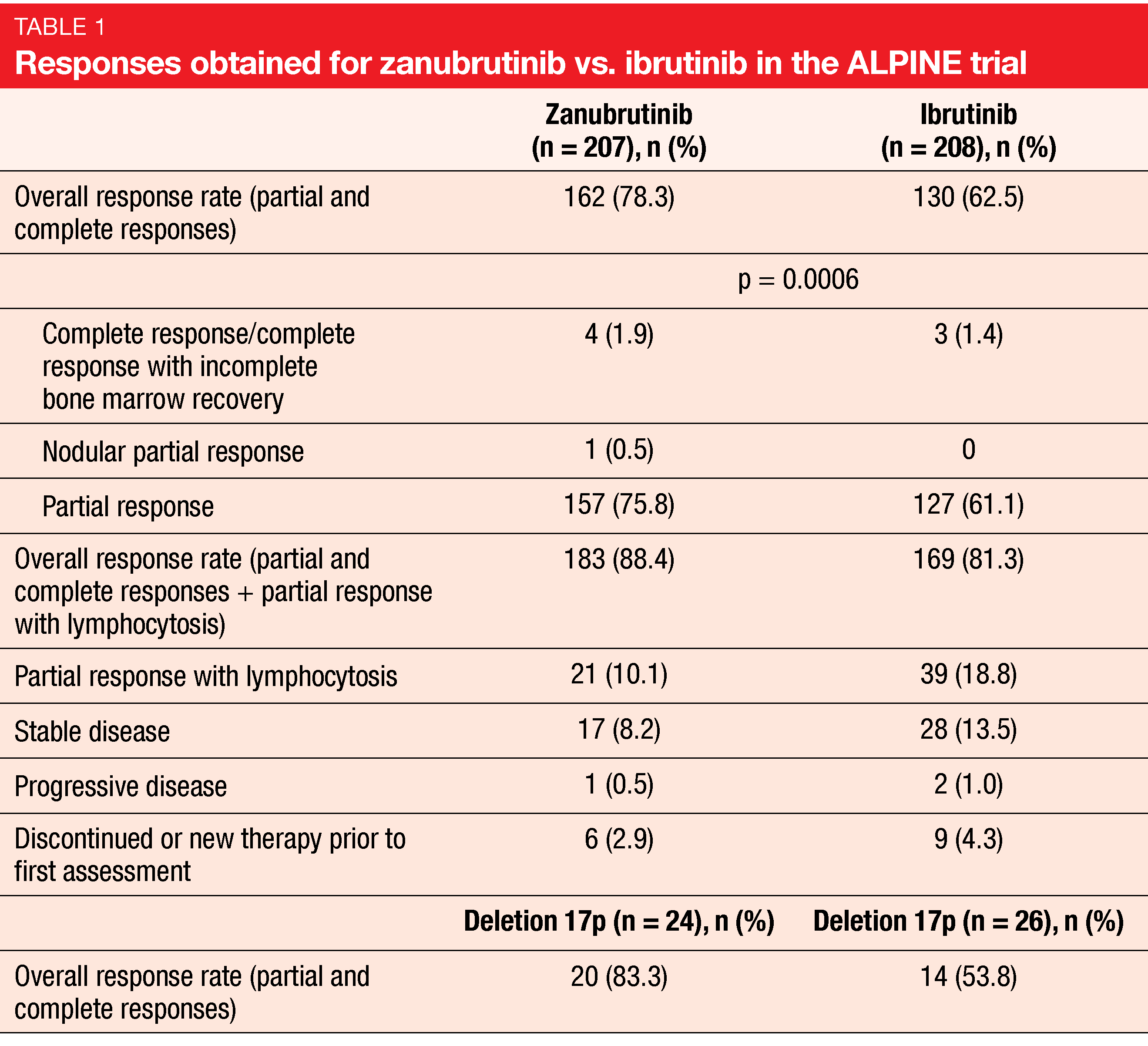
Indeed, zanubrutinib, as compared to ibrutinib, was shown to confer superior response, as well as other improvements. The ORR was 78.3 % and 62.5 % for zanubrutinib and ibrutinib, respectively (p = 0.0006; Table 1). Complete responses (CRs) and complete responses with incomplete bone marrow recovery (CRi) resulted in 1.9 % vs. 1.4 %. For the group that included patients with partial response and lymphocytosis in addition to those with CR and PR, the ORR was 88.4 % vs. 81.3 %. Likewise, patients with deletion 17p benefited from the new BTK inhibitor. According to the subgroup analysis, the ORR results for all key patient subgroups favored zanubrutinib.
Significant reduction in atrial fibrillation
With respect to progression-free survival (PFS), the patients treated in the experimental arm experienced a 60 % reduction in the risk of progression and death (HR, 0.40; p = 0.0007). The median PFS had not been reached yet in either arm; at 12 months, 94.9 % vs. 84.0 % were progression-free. Overall survival (OS) did not differ significantly, with 12-month OS rates of 97.0 % vs. 92.7 % (HR, 0.54; p = 0.1081).
A little over half of patients in both arms had grade ≥ 3 adverse events (AEs). Dose reductions and interruptions occurred with similar rates across the treatment arms, although AEs leading to treatment discontinuation were lower with zanubrutinib (7.8 % vs. 13.0 %). AEs on zanubrutinib therapy included mostly upper respiratory tract infections (21.6 %), neutropenia (19.6 %), and diarrhea (16.7 %). In the ibrutinib arm, diarrhea (19.3 %), neutropenia (15.5 %), anemia (15.0 %), upper respiratory tract infections and arthralgia (14.0 % each) prevailed. Arthralgia and muscle spasms were observed less frequently with zanubrutinib than with ibrutinib.
Among AEs of special interest, the incidence of any-grade atrial fibrillation was defined as a key secondary endpoint. Here, zanubrutinib offered a considerable advantage over ibrutinib (2.5 % vs. 10.1 %; p = 0.0014). No significant differences were noted regarding hemorrhage and hypertension. According to the authors, these data indicate that more selective BTK inhibition, with increases in terms of complete and sustained BTK occupancy, results in improved efficacy and safety outcomes.
Long-term findings from BGB-3111-205
The single-arm, multicenter, phase II BGB-3111-205 study has already demonstrated efficacy and tolerability of zanubrutinib 160 mg twice daily in relapsed/refractory CLL/SLL after ≥ 1 prior therapy [3]. According to the long-term results reported at EHA 2021 for 91 patients after a 34-month follow-up, responses deepened over time [4]. The updated ORR was 87.9 %, with 6.6 % of patients achieving CR. All subgroups analyzed responded to treatment including those with high-risk cytogenetics. Patients with deletion 17p/TP53 mutation and deletion 11q achieved high response rates of 91 % and 100 %, respectively.
The safety data were consistent with those previously reported. AEs led to treatment discontinuation in 15.4 %, and dose interruptions became necessary in 46.2 %. Among AEs of special interest, infections, neutropenia, and hemorrhage were observed most commonly. Two thirds of patients were still benefiting from continuous zanubrutinib treatment at the time of data cutoff.
Ibrutinib/venetoclax in fit patients: CAPTIVATE
The once-daily, all-oral, fixed-duration regimen of ibrutinib and the BCL2 inhibitor venetoclax has been shown to effectively induce tumor debulking in patients with CLL and to reduce the risk of tumor lysis syndrome (TLS) due to high tumor burden [5, 6]. In the first-line setting, the international, phase II CAPTIVATE trial is evaluating three cycles of ibrutinib lead-in followed by twelve cycles of ibrutinib/venetoclax. CAPTIVATE comprises the fixed-duration (FD) cohort and the minimal residual disease (MRD) cohort, which received MRD-guided randomization to either placebo vs. ibrutinib or ibrutinib vs. ibrutinib/venetoclax after the fixed-duration schedule. Results from the MRD cohort demonstrated undetectable MRD (uMRD) in more than two thirds of patients after 12 cycles of ibrutinib/venetoclax and 30-month PFS rates of ≥ 95 % irrespective of subsequent MRD-guided randomized treatment [6]. At EHA 2021, Allan et al. presented the primary analysis results for the FD cohort after a median follow-up of 14.0 months post completion of treatment [7].
The FD cohort contained 159 patients aged ≤ 70 years with previously untreated CLL/SCLL and ECOG performance status of 0–2. High-risk features including unmutated IGHV, deletion 17p/TP53 mutation and complex karyotype were present in 56 %, 17 %, and 19 %, respectively. Bulky disease was found in 30 %. The primary endpoint was the CR/CRi rate per investigator assessment in patients without deletion 17p; this cohort included 136 individuals.
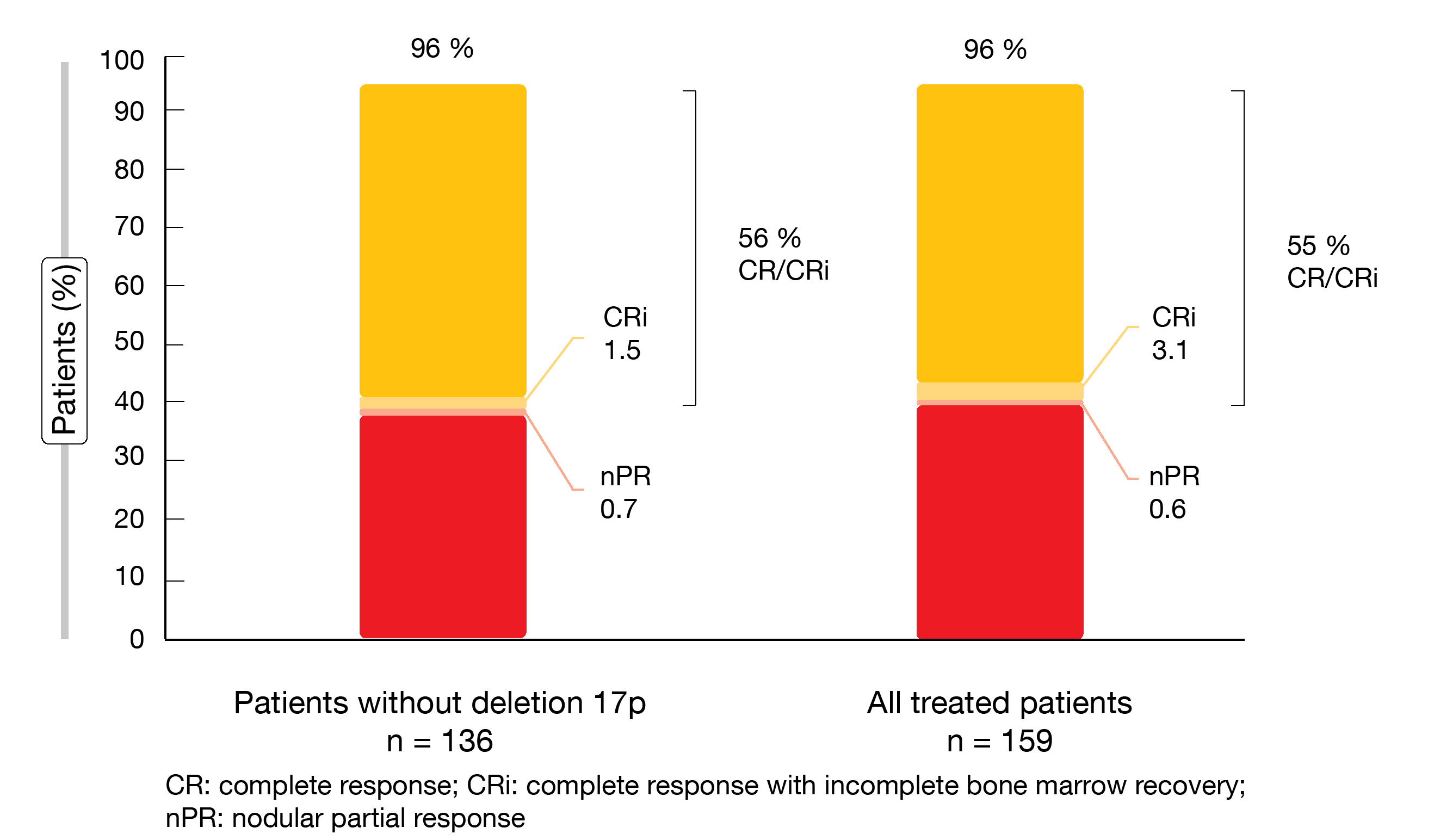
Fixed-duration treatment with ibrutinib/venetoclax was shown to induce deep and durable responses. With a 56 % CR/CRi rate, the primary endpoint was met (Figure 1). This provided a meaningful improvement over the 40 % rate obtained with the historical comparator of fludarabine, cyclophosphamide and rituximab (FCR) in the CLL10 study [8]. Best overall response amounted to 96 % both in patients without deletion 17p (i.e., those included in the primary endpoint analysis) and in all patients treated in the FD cohort (Figure 1). Complete responses lasted for ≥ 12 cycles in 87 % and 89 %, respectively. High CR/CRi rates were observed in almost all treated patients, including those with high-risk features. An exception was the group with bulky disease that showed a 31 % CR rate (vs. 66 % in patients without bulky disease).
Figure 1: CAPTIVATE: best overall responses for fixed-duration ibrutinib/venetoclax in patients without deletion 17p and in the total treated population
Deep and durable responses
As many as 76 % and 77 % of patients in the two cohorts achieved uMRD in the peripheral blood as best uMRD response; for the bone marrow, this was 62 % and 60 %, respectively. Despite the divergence of CR rates between patients with and without bulky disease, uMRD rates were similar across these groups. On the other hand, those with unmutated IGHV had higher uMRD rates than those with mutated IGHV. At 24 months, 98 % of patients in both groups were alive, and 96 % and 95 % among those without deletion 17p and all treated patients, respectively, showed freedom from progression. PFS rates at 24 months were high for patients with both unmutated and mutated IGHV (93 % and 97 %, respectively). In the cohort harboring deletion 17p/TP53 mutation, 56 % achieved CR/CRi, and best uMRD rates in peripheral blood and bone marrow amounted to 81 % and 41 %, respectively. Ninety-six percent of these patients were alive at 24 months, with 84 % being progression-free. To date, eight patients enrolled in CAPTIVATE have been retreated with single-agent ibrutinib. Six of them responded with partial remissions, and in two patients, response evaluation was pending at the time of data presentation.
The fixed-duration regimen proved tolerable. Ninety-two percent of patients completed the full schedule. Three cycles of ibrutinib were shown to provide effective tumor debulking; no clinical TLS occurred, and no patient had laboratory TLS per Howard criteria. Diarrhea (62 %), nausea (43 %), neutropenia (42 %), and arthralgia (33 %) emerged as the most common AEs. Any-grade atrial fibrillation and major hemorrhage were seen in 4 % and 2 %, respectively. Ibrutinib/venetoclax was well tolerated with concomitant medications including antihypertensive agents, acid-reducing drugs, antiplatelet agents, and anticoagulants. AEs leading to dose reduction occurred in 21 %; here, 88 % of patients had resolution of these AEs at the time of the analysis. The treatment was discontinued due to AEs in 5 %.
In their conclusions, the researchers noted that the results from the FD cohort are largely consistent with the MRD cohort [6] and support fixed-duration treatment with ibrutinib and venetoclax as an all-oral, once-daily, chemotherapy-free regimen that drives deep and durable responses.
GLOW study: ibrutinib/venetoclax in the elderly
The randomized, phase III GLOW study was specifically designed to assess the efficacy and safety of ibrutinib/venetoclax compared to chlorambucil/obinutuzumab in older or unfit patients with previously untreated CLL. These were ≥ 65 years of age or < 65 years with a CIRS (Cumulative Illness Rating Scale) score > 6 or creatinine clearance < 70 mL/min. No deletion 17p or known TP53 mutation were present.
In the experimental arm, 106 patients received ibrutinib/venetoclax for twelve cycles after a 3-cycle lead-in with ibrutinib. Patients in the control arm (n = 105) were treated with chlorambucil for six cycles plus obinutuzumab in cycles 2-6. The median age of the total patient population was 71 years, with one third in both arms being 75 years of age or older. CIRS scores > 6 were present in 69.8 % and 58.1 % in the experimental and control arms, respectively. Kater et al. reported the primary results of the GLOW study at EHA 2021 [9].
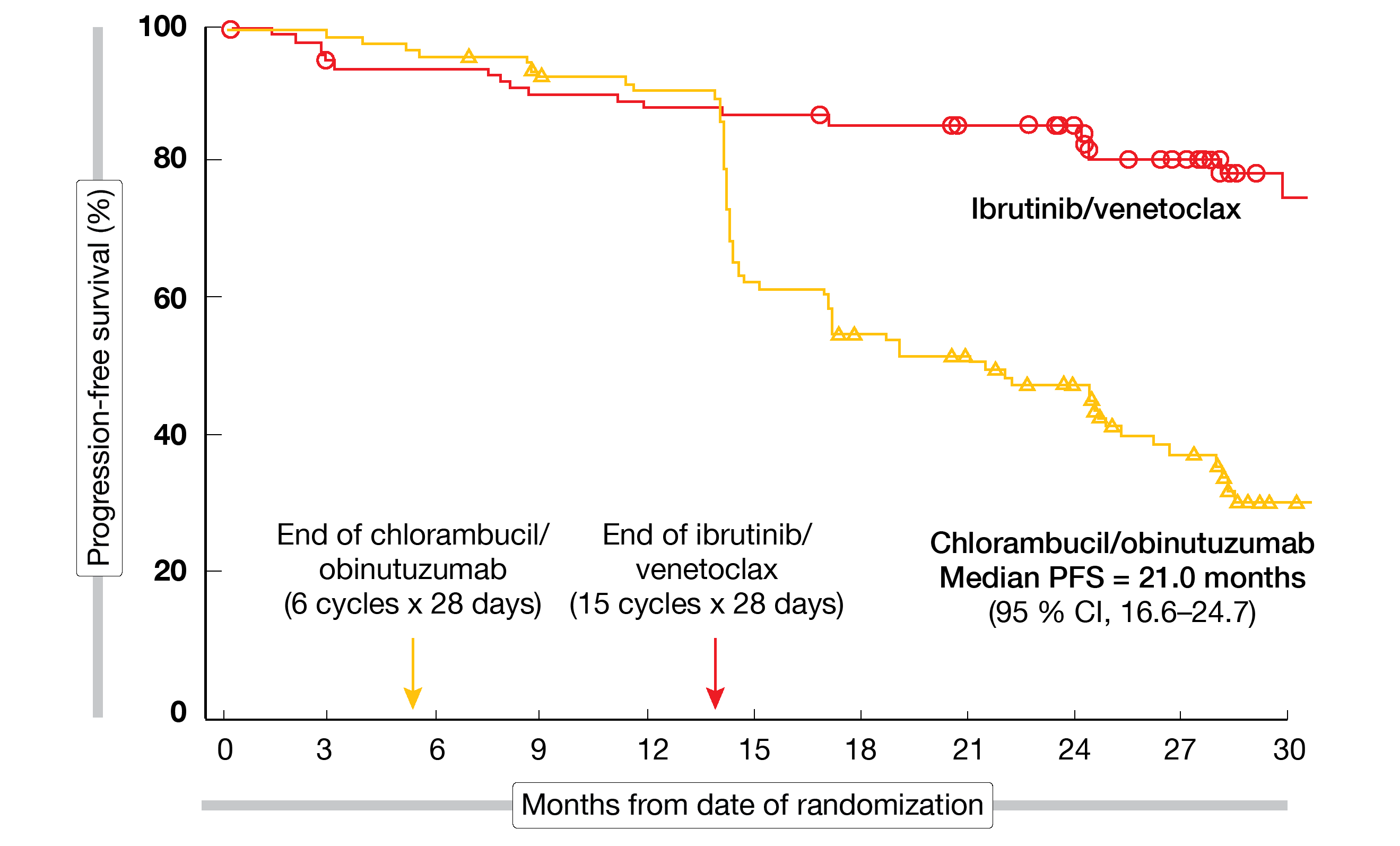
PFS by independent review committee (IRC) was defined as the primary endpoint. After a median follow-up of 27.7 month, ibrutinib/venetoclax, as compared to chlorambucil/obinutuzumab, gave rise to a 78 % reduction in the risk of progression and death (median PFS, not reached vs. 21.0 months; HR, 0.216; p < 0.0001; Figure 2). PFS improvement was consistent across the pre-specified subgroups. Ibrutinib/venetoclax induced significantly higher CR/CRi rates (38.7 % vs. 11.4 %; p < 0.0001). Moreover, responses were more durable, with 24-month rates of 90 % vs. 41 % in initial responders.
Figure 2: Progression-free survival advantage for ibrutinib/venetoclax vs. chlorambucil/obinutuzumab in older and comorbid patients treated in GLOW
Tripling of uMRD rate in the marrow
Three months after the end of treatment, the uMRD rate was significantly higher for ibrutinib/venetoclax vs. chlorambucil/obinutuzumab, particularly in the bone marrow (51.9 % vs. 17.1 %; p < 0.0001), but also in the peripheral blood (54.7 % vs. 39.0 %; p = 0.0259). With respect to best uMRD rates by flow cytometry, patients treated with the BTK-inhibitor–based regimen fared better. This applied to both bone marrow (67.9 % vs. 22.9 %) and peripheral blood (80.2 % vs. 46.7 %). Also, the marrow/blood uMRD concordance rate was comparatively higher in the experimental arm (92.9 % vs. 43.6 %), and the majority of ibrutinib-treated patients sustained uMRD in the peripheral blood 12 months after treatment cessation (84.5 %), while this was 29.3 % with the comparator regimen. Time to next treatment was substantially prolonged in the experimental arm. Ibrutinib/venetoclax reduced the risk of requiring second-line therapy by 86 % (HR, 0.143).
The tolerability profiles for both regimens were consistent with the observations in the setting of CLL treatment in elderly comorbid patients. Serious AEs that occurred in ≥ 5 % of cases included infections (12.3 % vs. 8.6 %) and atrial fibrillation (6.6 % vs. 0 %). Two patients in the experimental arm (1.9 %) discontinued ibrutinib due to atrial fibrillation. After three cycles of ibrutinib lead-in, less than 2 % of patients remained at risk for TLS based on high tumor burden. Causes of death were generally similar across the arms, with infections and cardiac events being most common. As the authors noted in their conclusions, the results of the GLOW study support the positive clinical profile of all-oral, once-daily, fixed-duration ibrutinib/venetoclax as first-line treatment for older patients with CLL.
CLL14: 4-year update
Patients with previously untreated CLL and coexisting medical conditions (CIRS > 6 and/or creatinine clearance < 70 mL/min) were enrolled in the CLL14 study that compared venetoclax/obinutuzumab with chlorambucil/obinutuzumab for six cycles. After the combination phase, venetoclax and chlorambucil as monotherapies were administered for another six cycles in the experimental and control arms, respectively. Each arm contained 216 patients. The median total CIRS scores were 9 and 8 for venetoclax/obinutuzumab and chlorambucil/obinutuzumab, respectively. Approximately 60 % of patients across the groups had an unmutated IGHV status, and 12 % each showed TP53 deletions and/or mutations. Al-Sawaf et al. reported the 4-year update of the CLL14 study at the EHA 2021 congress [10].
According to this, very low rates of grade ≥ 3 AEs resulted after cessation of treatment in both arms. No long-term or late-onset AEs emerged, which suggests benefits due to lower risk of toxicity and drug-drug interactions based on the fixed-duration approach. Similar proportions of patients developed at least one secondary primary malignancy (14 % vs. 18.9 %). PFS was defined as the primary endpoint of the study. After the prolonged follow-up, median PFS had not been reached with venetoclax/obinutuzumab and was 36.4 months with chlorambucil/obinutuzumab (HR, 0.33; p < 0.0001). Three years after treatment cessation, 74.0 % vs. 35.4 % of patients remained progression-free.
PFS assessment according to TP53 and IGHV status showed substantial benefit from venetoclax treatment in patients with TP53 deletions/mutations and unmutated IGHV, although PFS was still shorter than in those without TP53 deletions/mutations and with mutated IGHV. This demonstrates that patient outcomes can be improved with venetoclax/obinutuzumab in the presence of high-risk features, although these cannot be overcome. Head-to-head comparisons with continuous treatment regimens are called for to elucidate the optimal strategy in these subgroups.
MRD dynamics favor venetoclax/obinutuzumab
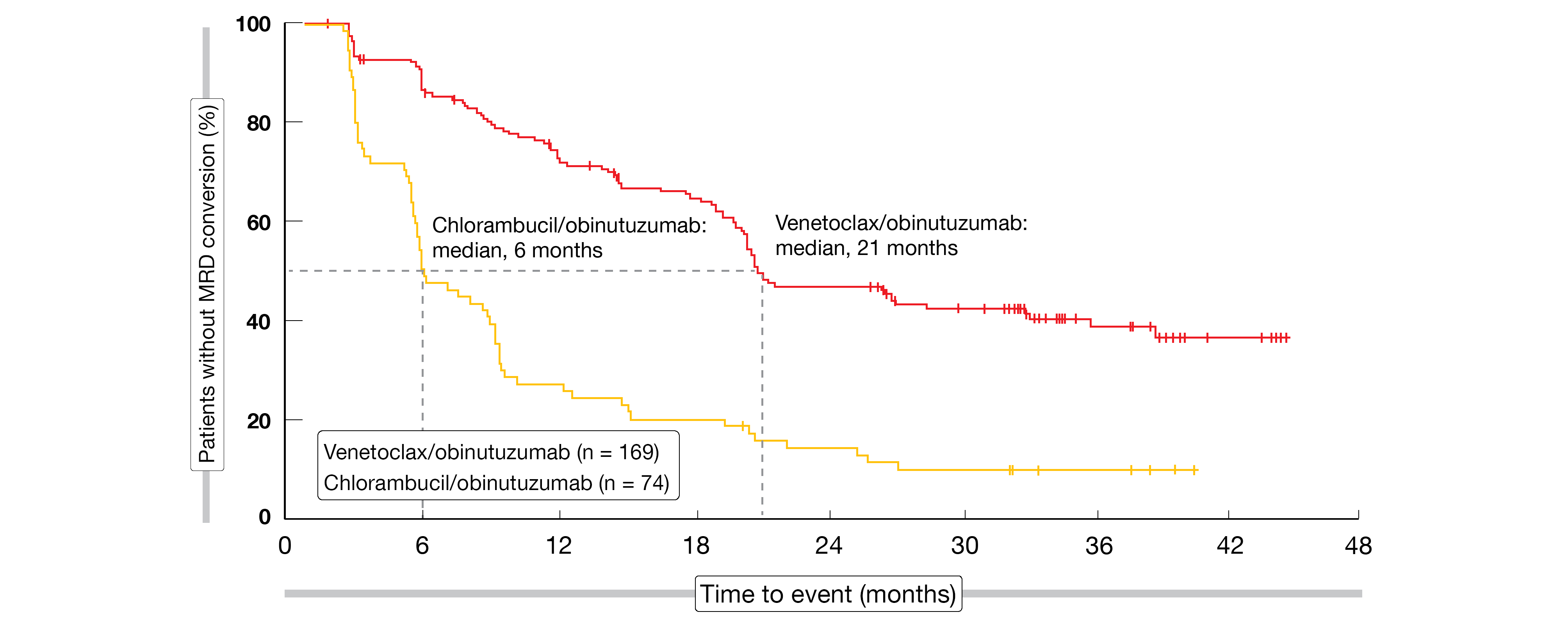
Time to next treatment had not been reached in either arm at the time of the analysis; at 4 years, the rates were 81.08 % vs. 59.9 % (HR, 0.46; p < 0.0001). Considerably fewer subsequent anti-leukemic therapies were administered in the venetoclax/obinutuzumab-treated group (17 vs. 70). No overall survival difference has been noted to date (4-year OS rates, 85.3 % vs. 83.1 %). CLL-related deaths occurred only in seven patients in the experimental arm, which indicates that the treatment is able to mitigate the disease-inherent risk. MRD was maintained more effectively with venetoclax/obinutuzumab according to longitudinal assessments, although the data are not mature here. Loss of uMRD response occurred after a median of 21 months after discontinuation of treatment with the venetoclax-based therapy, while this was 6 months for chlorambucil/obinutuzumab (Figure 3).
A population-based MRD model was conducted to evaluate growth dynamics in the CLL14 study as this enables description of differential MRD growth trajectories [11]. The findings showed that MRD doubling time was significantly longer with venetoclax/obinutuzumab than with chlorambucil/obinutuzumab (80 vs. 69 days; p < 0.0039), as was time to MRD 10-2 (1,259 vs. 233 days; p < 2e-16). In addition to other factors such as genetic aberrations, disease burden and risk according to the international prognostic index, the regimens themselves were identified as factors affecting the length of MRD doubling time and time to MRD 10-2. In their conclusions, the authors noted that MRD eradication is significantly more effective with venetoclax/obinutuzumab than with chlorambucil/obinutuzumab, and MRD regrowth is significantly slower after cessation of the venetoclax-based regimen. This translated into a sustained and significant PFS benefit for patients with MRD doubling time > 76 days vs. those with MRD doubling time < 76 days.
Figure 3: Time to MRD conversion from > 10-4 at the end of treatment with venetoclax/obinutuzumab vs. chlorambucil/obinutuzumab
Impact of genetic markers in CLL14
Another analysis explored the impact of genetic markers on patient outcomes observed in the CLL14 study [12]. This confirmed the IGHV status as a prognostic marker, as patients with unmutated IGHV experienced shorter PFS in both arms, although the impact was less pronounced with the venetoclax-based treatment (HRs, 2.14 vs. 3.07). Overall, the experimental treatment was superior in patients with both unmutated and mutated IGHV (HRs, 0.25 and 0.36, respectively). The same was true for deletion 17p that adversely affected PFS in both arms (HRs, 3.19 and 3.15, respectively), while venetoclax/obinutuzumab was superior to the chlorambucil-based regimen independent of the presence of 17p deletion.
Chlorambucil-treated patients with deletion 11q experienced shorter PFS compared to those without deletion 11q (HR, 1.84), whereas no difference was observed for those on venetoclax/obinutuzumab therapy. This also applied to BIRC3 mutations. Acquisition of deletion 17p or 11q after therapy occurred more frequently in the control arm, while patients in the experimental arm mainly showed decreasing deletion 17p and 11p fractions.
For TP53 and BIRC3 mutations, the variant allele frequency basically decreased with venetoclax/obinutuzumab over time but remained stable or increased with chlorambucil/obinutuzumab. In the absence of deletion 17p, TP53 major and minor mutations had no impact on PFS. The researchers also performed an analysis of resistance mutations in 113 relapse samples. Here, venetoclax-treated patients showed no acquired mutations in BCL2, BIM, BAX, BCL-XL or MCL1, which indicated that resistance to venetoclax is unlikely to occur in a fixed-duration setting. At the same time, new high-risk mutations such as TP53, BIRC3, SF3B1 and ATM occurred more frequently with chlorambucil.
ELEVATE-RR: acalabrutinib vs. ibrutinib
The next-generation, potent, highly selective BTK inhibitor acalabrutinib offers decreased alternative-target activity compared to ibrutinib in vitro, which potentially confers an improved tolerability profile [13, 14]. ELEVATE-RR is the first head-to-head trial to compare acalabrutinib with ibrutinib in patients with previously treated CLL and deletion 17p or deletion 11q. This open-label, phase III study was conducted at 124 centers in 15 countries. Overall, 533 patients were randomized to either acalabrutinib 100 mg twice daily (n = 268) or ibrutinib 420 mg/d (n = 265). Non-inferiority of IRC-assessed PFS constituted the primary outcome.
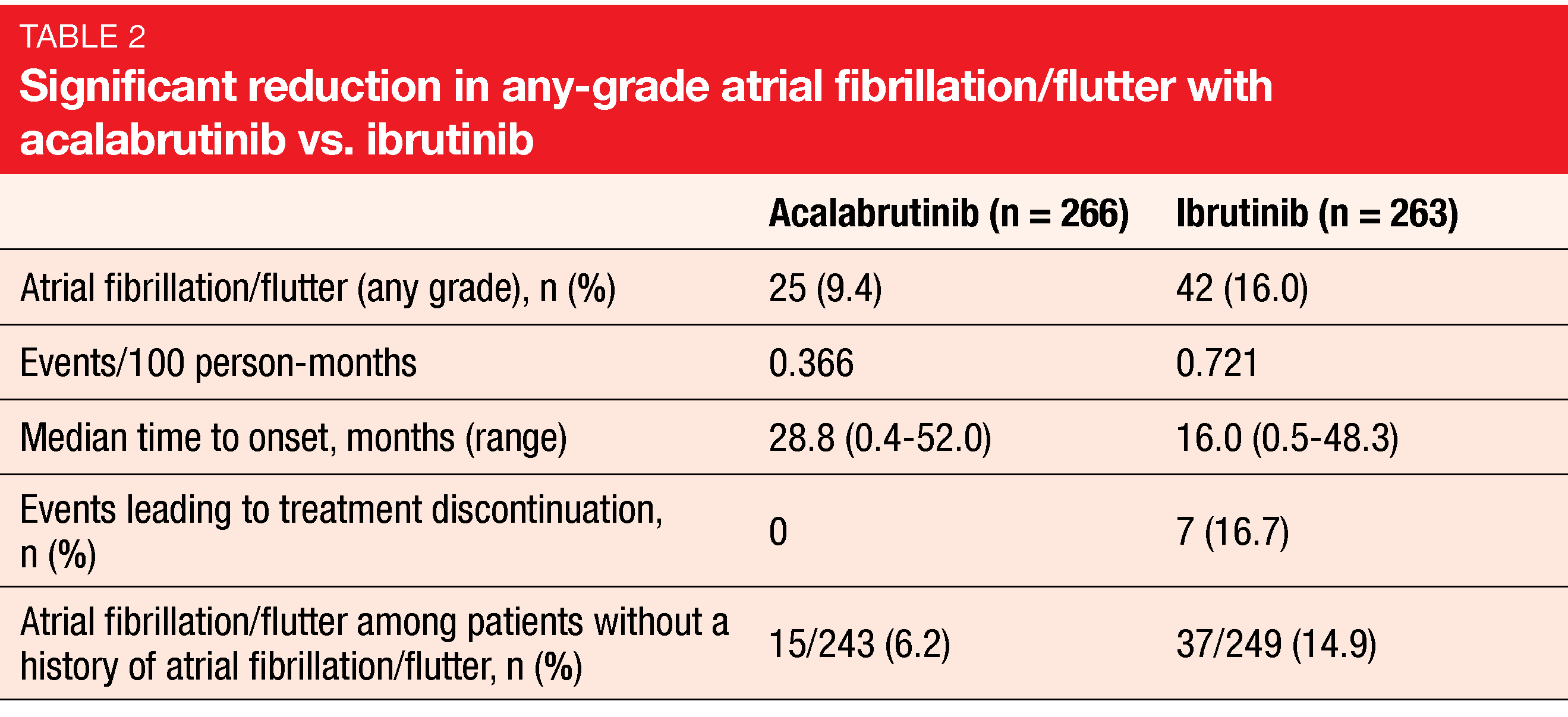
According to the findings presented at EHA 2021, the trial met its primary endpoint, with median PFS of 38.4 months in both arms (HR, 1.00) [15]. IRC-assessed PFS was comparable across prespecified subgroups. A key secondary endpoint related to the incidence of any-grade atrial fibrillation or flutter. Here, acalabrutinib demonstrated significantly improved results with an absolute reduction of 6.6 % in any-grade incidence rates (Table 2). Markedly fewer patients in the experimental arm who had no prior history of atrial fibrillation or flutter experienced new events. No differences were observed for grade ≥ 3 infection (30.8 % vs. 30.0 %) and Richter’s transformation (3.8 % vs. 4.9 %). Median OS had not been reached in either arm; the mortality risk was 18 % lower with acalabrutinib (HR, 0.82).
Compared to ibrutinib, acalabrutinib demonstrated lower rates of grade ≥ 3 AEs (68.8 % vs. 74.9 %), serious AEs (53.8 % vs. 58.6 %) and treatment discontinuation due to AEs (14.7 % vs. 21.3 %). While headache and cough occurred more commonly with acalabrutinib, ibrutinib shower higher rates of diarrhea, arthralgia, and hypertension. Bleeding events occurred in 38.0 % vs. 51.3 % and interstitial lung disease/pneumonitis in 2.6 % vs. 6.5 %. Cumulative incidences of any-grade atrial fibrillation/flutter and hypertension over time were lower for acalabrutinib with HRs of 0.52 and 0.34, respectively. This also applied to bleeding events, diarrhea, and arthralgia (HRs, 0.63, 0.61 and 0.61, respectively). The authors stated in their summary that acalabrutinib is better tolerated and has similar efficacy compared to ibrutinib in patients with previously treated CLL.
Four-year data from ELEVATE-TN
The pivotal phase III ELEVATE-TN study investigated the combination of acalabrutinib and obinutuzumab (A+O; n = 179), as well as acalabrutinib monotherapy (A; n = 179), in treatment-naïve patients aged ≥ 65 years or 18-64 years with comorbidities (i.e., creatinine clearance 30-69 mL/min or CIRS-G score > 6). Patients in the control arm (n = 179) received obinutuzumab plus chlorambucil (O+Clb). Early results at a median follow-up of 28.3 months demonstrated superior efficacy of acalabrutinib ± obinutuzumab with an acceptable tolerability profile [16]. At EHA 2021, Sharman et al. reported the 4-year update [17].
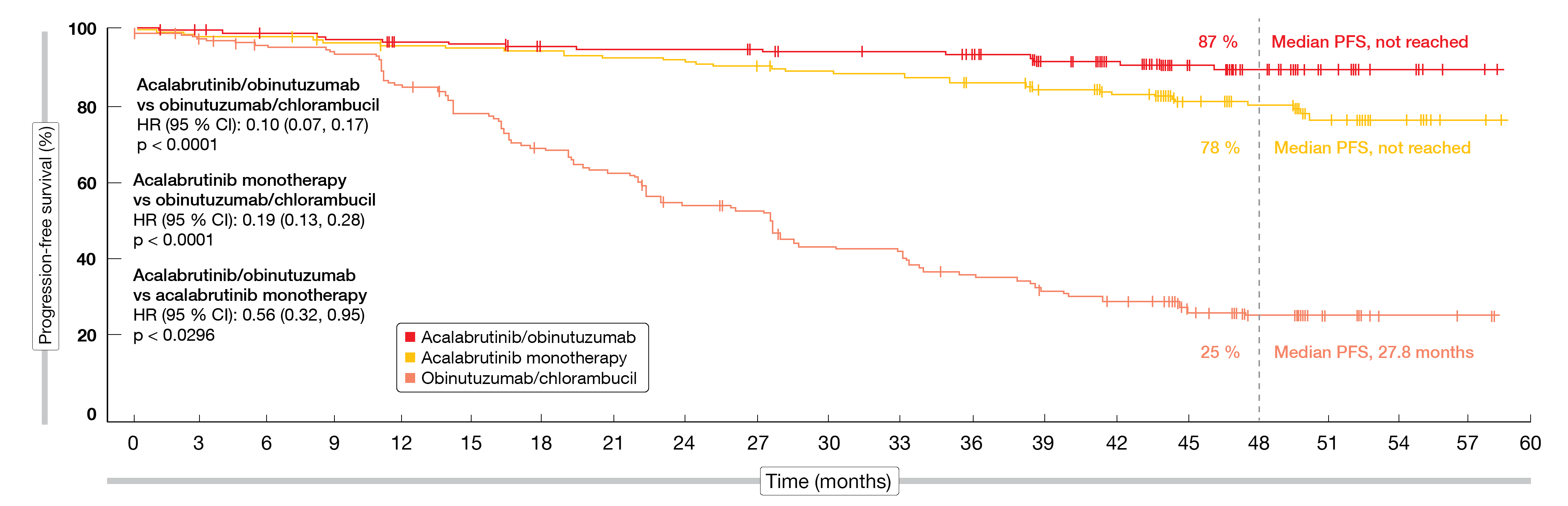
According to this analysis, the efficacy and safety of the acalabrutinib-based therapy was maintained relative to O+Clb. At a follow-up of 46.9 months, treatment was ongoing in 74.9 % and 69.3 % of patients in the A+O and A arms, respectively, but in none in the O+Clb arm. Median PFS was significantly longer for A+O vs. O+Clb (HR, 0.10; p < 0.0001) and A vs. O+Clb (HR, 0.19; p < 0.0001; Figure 4). Median PFS had not been reached in the acalabrutinib arms and was 27.8 months for obinutuzumab/chlorambucil. The PFS rates at 48 months amounted to 87 %, 78 % and 25 %, respectively; here, the analysis revealed a trend in favor of A+O over A.
The PFS findings were consistent across high-risk genetic subgroups. Patients with deletion 17p/TP53 mutation showed 48-month PFS rates of 76 % (HR, 0.17 vs. O+Clb; p < 0.0001), 75 % (HR, 0.18 vs. O+Clb; p < 0.0001), and 18 %, respectively. In the group with unmutated IGHV, this was 86 % (HR, 0.06 vs. O+Clb; p < 0.0001), 77 % (HR, 0.10; p < 0.0001), and 4 %, respectively. The CR/CRi rates had increased from the time of the interim analysis at 28.3 months [16] and now were 30.7 %, 11.2 % and 13.0 % across the three arms. Overall, 96.1 %, 89.9 %, and 82.5 % of patients responded. Those with CR/CRi showed uMRD in 38 %, 10 %, and 9 %, respectively. For OS, the findings yielded a trend in favor of A+O as compared to O+Clb (HR, 0.50; p = 0.0604). In the A+O arm, 93 % of patients were alive at 48 months, while this applied to 88 % in the other groups.
The safety of both acalabrutinib-based regimens was consistent with the interim findings, including low incidences of cardiovascular events such as atrial fibrillation and hypertension, and low rates of treatment discontinuation despite longer treatment exposure. Grade ≥ 3 infections occurred more frequently with A+O than with A monotherapy (23.6 % and 16.2 %, respectively). According to the authors, acalabrutinib with or without obinutuzumab demonstrated durable disease control, tolerability, and flexibility to tailor treatment as monotherapy or combination therapy in treatment-naïve CLL patients.
Figure 4: Superior progression-free survival with acalabrutinib/obinutuzumab and acalabrutinib monotherapy vs. obinutuzumab/chlorambucil
CLL2-BAAG: bendamustine, VenG & acalabrutinib
In the CLL2-BAG trial, bendamustine followed by obinutuzumab and venetoclax induced ORR and uMRD rates of 95 % and 87 %, respectively [18]. As a relevant proportion of patients retained residual lymphadenopathy and only achieved partial remission, it was decided to add a BTK inhibitor in the next phase II study. At EHA 2021, Cramer et al. reported results from the ongoing CLL2-BAAG trial that is evaluating bendamustine followed by obinutuzumab, acalabrutinib and venetoclax [19].
Debulking with two cycles of bendamustine is recommended for patients with high tumor burden prior to induction that contains obinutuzumab monotherapy in the first cycle and obinutuzumab plus acalabrutinib in cycles 2-6. Venetoclax is administered at the full dose in cycles 4-6 after the ramp-up in cycle 3. The uMRD rate at the end of the six induction cycles constitutes the primary endpoint of the CLL2-BAAG trial. Maintenance is open to responders and includes daily acalabrutinib and venetoclax plus 3-monthly obinutuzumab for a maximum of eight cycles. Forty-five patients with relapsed/refractory CLL were enrolled. Among these, 32 % had 17p deletion/TP53 mutation, and in 30 %, a complex karyotype was present. Forty-seven percent had already received targeted agents, such as ibrutinib, venetoclax, or both.
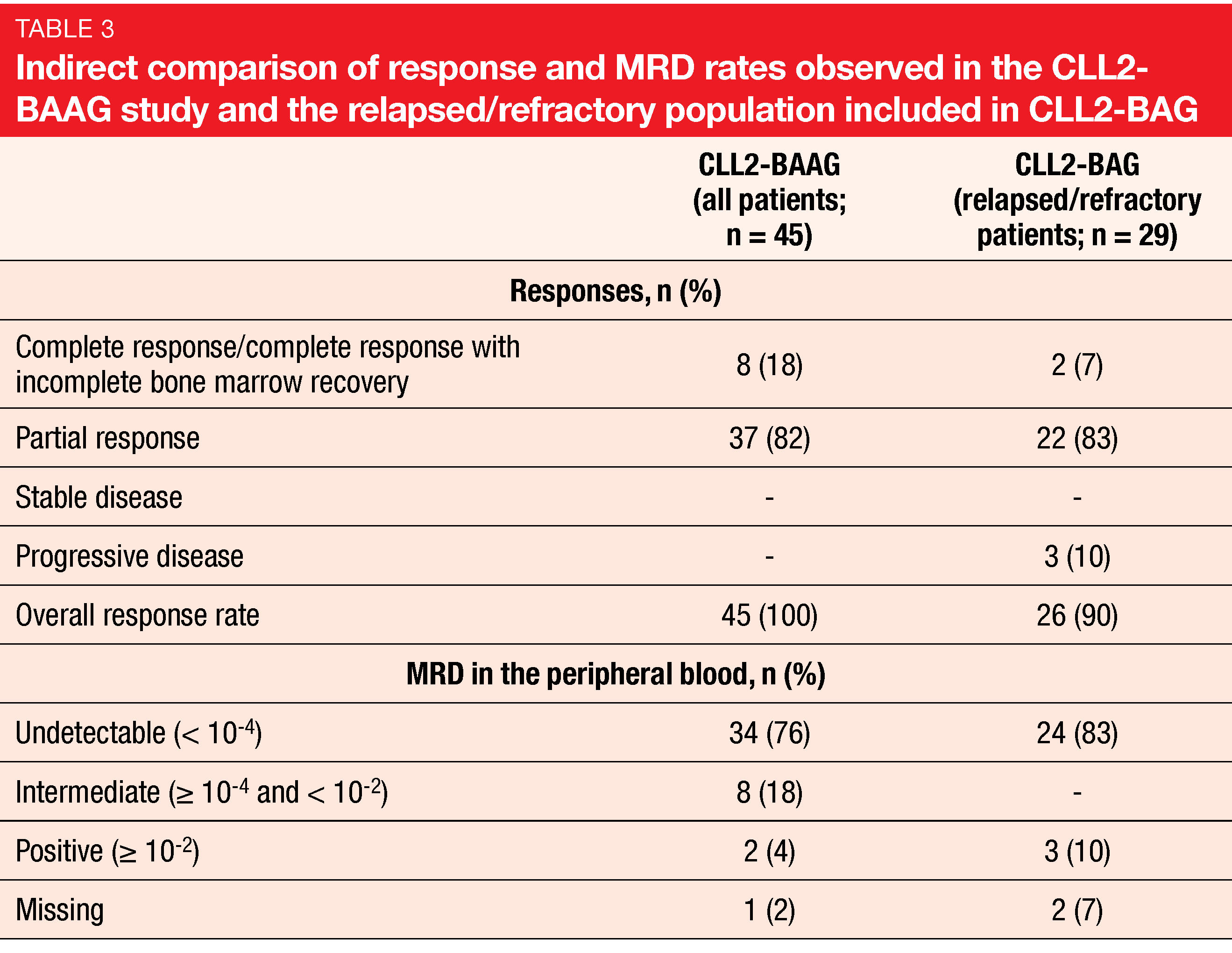
uMRD rate of 76 %
All patients responded to treatment. At the end of the induction phase, the CR/CRi rate was 18 %, and partial remission had been obtained in 82 % (Table 3). An uMRD rate of 76 % was found in the peripheral blood. This fell slightly short of the study assumption based on the 83 % uMRD rate observed in the relapsed/refractory population of the CLL2-BAG trial [18]. Therefore, the primary endpoint of CLL2-BAAG was not met. Nevertheless, considering the adverse genetic setup in one third of the population and pretreatment with targeted agents in half of the cases, the authors deemed this outcome impressive. Moreover, a redistribution phenomenon caused by the BTK inhibitor might be responsible for the lower rate, and improvement with continued follow-up can be expected.
Until data cutoff, maintenance treatment had been started in 32 patients (71 %). Twelve of these have discontinued, among them 9 (28 %) who stopped treatment due to uMRD. The regimen of bendamustine followed by obinutuzumab, acalabrutinib and venetoclax did not give rise to cumulative or unexpected toxicity. Overall, these findings are in accordance with other phase II trials investigating the triple combination. Phase III studies are ongoing.
Promising results for pirtobrutinib
The highly potent and selective non-covalent BTK inhibitor pirtobrutinib (LOXO-305) is being investigated in patients with previously treated advanced B-cell malignancies in the phase I/II BRUIN study. Roeker et al. presented the results from the CLL/SLL cohort at EHA 2021 [20]. The safety and efficacy populations included 170 and 139 patients, respectively. They had previously been treated with all classes of available therapies including anti-CD20 antibodies, BTK inhibitors, chemotherapy, BCL2 inhibitors and PI3K inhibitors. The median number of prior lines of therapy was 3.
In terms of pharmacokinetics, plasma exposures were shown to be dose-dependent and linear. A daily dose of 200 mg was selected as the recommended phase II dose. Responses were observed across all dose levels and irrespective of BTK C481 mutation status, the reason for prior BTK inhibitor discontinuation (i.e., progression vs. intolerance), or other classes of prior therapies including covalent BTK inhibitors, BCL2 inhibitors, and PI3Kδ inhibitors. The evaluable CLL/SLL population (n = 139) achieved an ORR of 63 %. In the BTK-pretreated cohort (n = 121), this was 62 %. ORR increased over time. At ≥ 10 months since the start of treatment, the rate had risen to 86 %. Only five responders discontinued treatment, which was due to disease progression in four cases and the decision to perform transplantation in one patient who was in partial remission.
The researchers demonstrated that notable covalent BTK-inhibitor–associated toxicities were only rarely observed, which was consistent with the design of pirtobrutinib as a highly selective and non-covalent BTK inhibitor. Longer follow-up is needed to better understand the safety profile of the agent in the setting of chronic administration. Overall, pirtobrutinib appeared well tolerated and exhibited promising efficacy in heavily pretreated CLL/SLL patients.
REFERENCES
- Tam CS et al., Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019; 134(11): 851-859
- Hillmen P et al., First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. EHA 2021, LB1900
- Xu W et al., Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol 2020; 13(1): 48
- Xu W et al., Zanubrutinib monotherapy in patients with relapsed or refractory chronic lymphocytic leukemia: 34-month follow-up results. EHA 2021, EP639
- Jain N et al., Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med 2019; 380(22): 2095-2103
- Wierda WG et al., Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma: 1-year disease-free survival results from the MRD cohort of the phase 2 CAPTIVATE study. ASH 2020, 123
- Allan JN et al., Primary analysis of the fixed-duration cohort from the phase 2 CAPTIVATE study of first-line ibrutinib + venetoclax for chronic lymphocytic leukemia/small lymphocytic lymphoma. EHA 2021, S147
- Eichhorst B et al., First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2016; 17(7): 928-942
- Kater AP et al., Fixed-duration ibrutinib plus venetoclax versus chlorambucil plus obinutuzumab for first-line treatment of chronic lymphocytic leukemia: primary analysis of the phase 3 GLOW study. EHA 2021, LB1902
- Al-Sawaf O et al., Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 4-year follow-up analysis of the randomized CLL14 study. EHA 2021, S146
- Al-Sawaf O et al., Venetoclax-obinutuzumab modulates clonal growth: results of a population-based minimal residual disease model from the randomized CLL14 study. ICML 2021, 031
- Tausch E et al., Genetic markers and outcome with front-line obinutuzumab plus either chlorambucil or venetoclax – updated analysis of the CLL14 trial. EHA 2021, S144
- Barf T et al., Acalabrutinib (ACP-196): A covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther 2017; 363(2): 240-252
- Byrd JC et al., Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016; 374(4): 323-332
- Hillmen P et al., First results of a head-to-head trial of acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia. EHA 2021, S145
- Sharman J et al., Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 2020; 395(10232): 1278-1291
- Sharman JP et al., Acalabrutinib ± obinutuzumab vs obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: ELEVATE-TN 4-year follow-up. EHA 2021, S148
- Cramer P et al., Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol 2018; 19(9): 1215-1228
- Cramer P et al., CLL2-BAAG, a prospective, open-label, multicenter phase II trial to evaluate the efficacy and safety of a sequential regimen of bendamustine followed by obinutuzumab, acalabrutinib and venetoclax in patients with relapsed/refractory CLL. ICML 2021, 034
- Roeker L et al., Pirtobrutinib (LOXO-305), a next generation, highly selective, non-covalent BTK inhibitor in previously treated CLL/SLL: results from the phase 1/2 BRUIN study. EHA 2021, EP633
© 2021 Springer-Verlag GmbH, Impressum