Extending anti-PD-1–based options in the setting of Hodgkin lymphoma
Patients with relapsed or refractory classical Hodgkin lymphoma (cHL) who have failed high-dose chemotherapy and autologous stem cell transplantation (HDT/ASCT) have poor prognosis, which also applies to those with chemotherapy-resistant disease who are ineligible for HDT/ASCT [1-4]. The presence of chromosome 9p24.1 alterations in cHL provides a rationale for immune checkpoint inhibition as this leads to overexpression of the PD-L1 ligands [5-7]. Anti-PD-1 antibodies including nivolumab, pembrolizumab, tislelizumab, camrelizumab and sintilimab have changed the treatment paradigm of adults with relapsed/refractory cHL [8-14]. Data presented at ASCO, EHA and ICML 2021 demonstrated efficacy of new agents and established PD-1 inhibitors over extended periods of time.
CheckMate 205
The pivotal, multicenter, single-arm, phase II CheckMate 205 trial evaluated the PD-1 inhibitor nivolumab at a dose of 3 mg/kg 2-weekly in patients with relapsed/refractory cHL after ASCT failure in three cohorts. Cohort A included 63 brentuximab vedotin(BV)-naïve patients; those in Cohort B (n = 80) had received BV after ASCT, while those in Cohort C (n = 100) had been treated with BV before and/or after ASCT. Objective response rate (ORR) by independent review committee (IRC) was defined as the primary endpoint. In Cohort C, patients who achieved complete remission (CR) for ≥ 1 year could discontinue treatment and receive re-treatment if relapses occurred within two years.
At the time of the 33-month follow-up presented at the ASH 2018 Congress, the ORR was 71 %, with 21 % of patients obtaining CR [15]. Ansell et al. reported updated results from CheckMate 205 with a median follow-up of 58 months at ICML 2021 [16]. Median duration of treatment was 14 months in the entire cohort. In 24 %, patients had been able to receive subsequent hematopoietic stem cell transplant based on the benefit from the study treatment.
Response-related outcome improvements
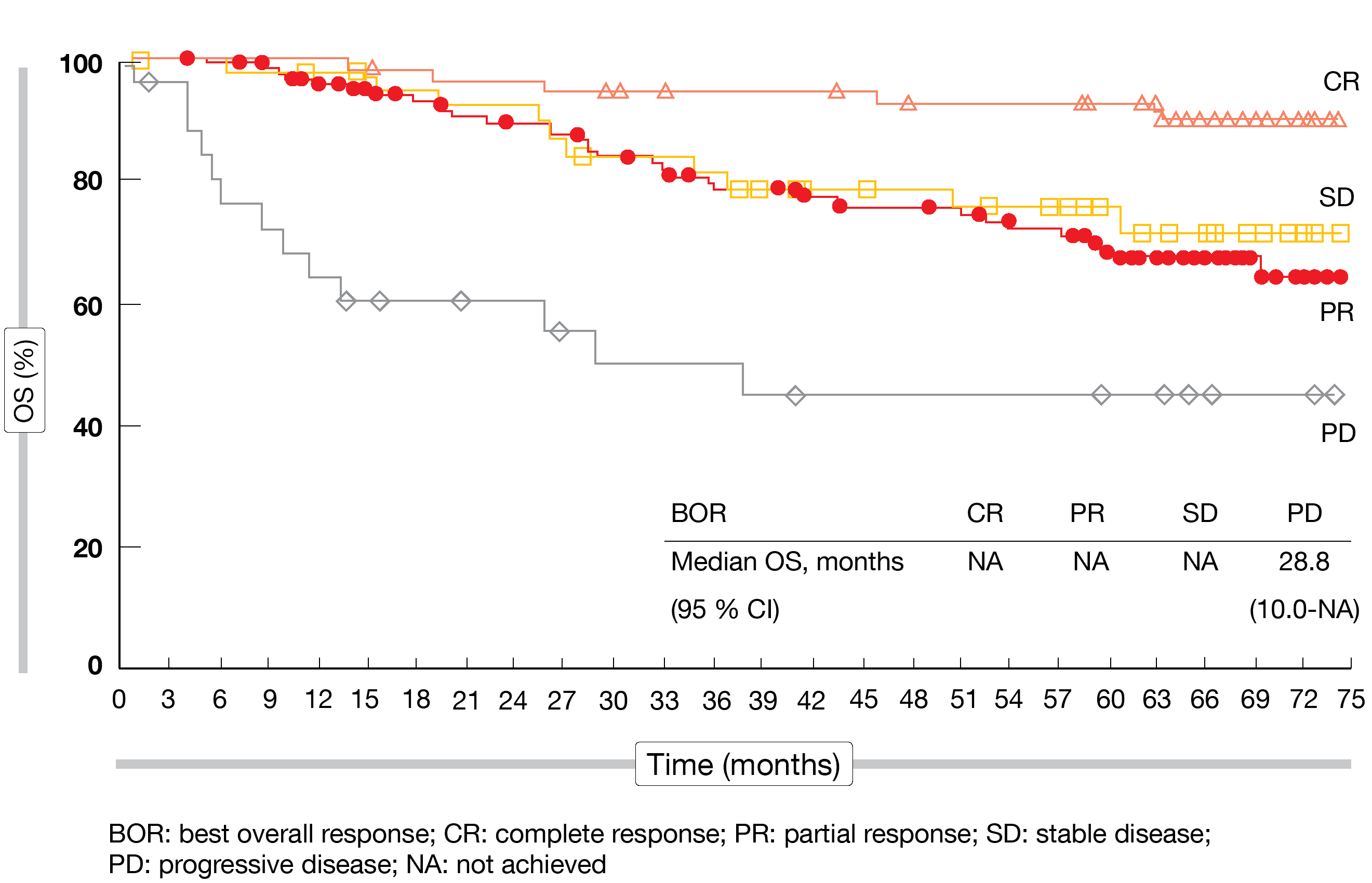
The 5-year follow-up confirmed the efficacy and safety of nivolumab in patients with cHL who have progressed or relapsed after ASCT. ORRs were 65 %, 71 % and 75 % for Cohorts A, B and C, respectively. Complete remissions resulted in 32 %, 14 % and 21 %, respectively. The CR rate in the total population had increased from 16 % at 33 months to 21 % at 58 months. Remissions proved durable, particularly in patients who had obtained CR; here, the median duration of response was 30.3 months compared to 13.5 months in the group with partial remission (PR). Favorable overall survival (OS) results were observed over time. At 5 years, 71.4 % of all patients were alive, and median OS had not been reached in the groups with CR, PR, and stable disease, with those in the CR cohort showing the best survival outcome (Figure). Even in the group that progressed during the study and received salvage therapies, many patients survived.
The 5-year progression-free survival (PFS) rate in the total population amounted to 18 %, with median PFS of 15.1 months. Median PFS was longest in the CR cohort (37.4 months); this was more than double the result seen for patients with PR (15.2 months).
The adverse event (AE) profile resembled the findings reported previously. No new safety signals or increases in toxicity were noted over time. Any-grade serious treatment-related AEs (TRAEs) occurred in 15 %, and the treatment was discontinued due to TRAEs in 11 %. Immune-related AEs (irAEs) were mainly classified as grade 1 or 2. Rash occurred most frequently (12 %), followed by hepatitis (6 %), and pneumonitis (6 %). Overall, these proportions were relatively modest despite the patients being on treatment for a long time.
In Cohort C, 12 patients out of 100 discontinued nivolumab therapy after they had maintained CR for ≥ 1 year. Among these, six remained in CR with no additional intervention. Four of the other six patients experienced disease progression. Three were re-treated per protocol, with one continuing on treatment and being in remission at the time of the analysis. In their summary, the authors pointed out that it appears feasible to stop nivolumab therapy after one year of CR and to re-initiate treatment upon disease progression.
Figure: Overall survival by best response obtained with nivolumab in CheckMate 205
BGB-A317-203: tislelizumab
Optimized efficacy can be expected from the PD-1 inhibitor tislelizumab that was engineered to minimize binding to FcγR on macrophages, which compromises anti-tumor activity of PD-1 antibodies through activation of antibody-dependent macrophage-mediated killing of effector T cells [17, 18]. The pivotal, multicenter, single-arm, phase II BGB-A317-203 trial tested tislelizumab 200 mg i. v. every 3 weeks in 70 patients with relapsed/refractory cHL who were ineligible for intensive treatment after ≥ 2 lines of systemic therapy or had achieved no response or progressed after HDT/ASCT. All patients had received chemotherapy; immunotherapy and BV had been administered in 21.4 % and 5.7 %, respectively. Nineteen percent had undergone ASCT, while the remaining patients were no candidates for transplant. The ORR by IRC constituted the primary endpoint. Song et al. presented long-term data at EHA 2021 [19].
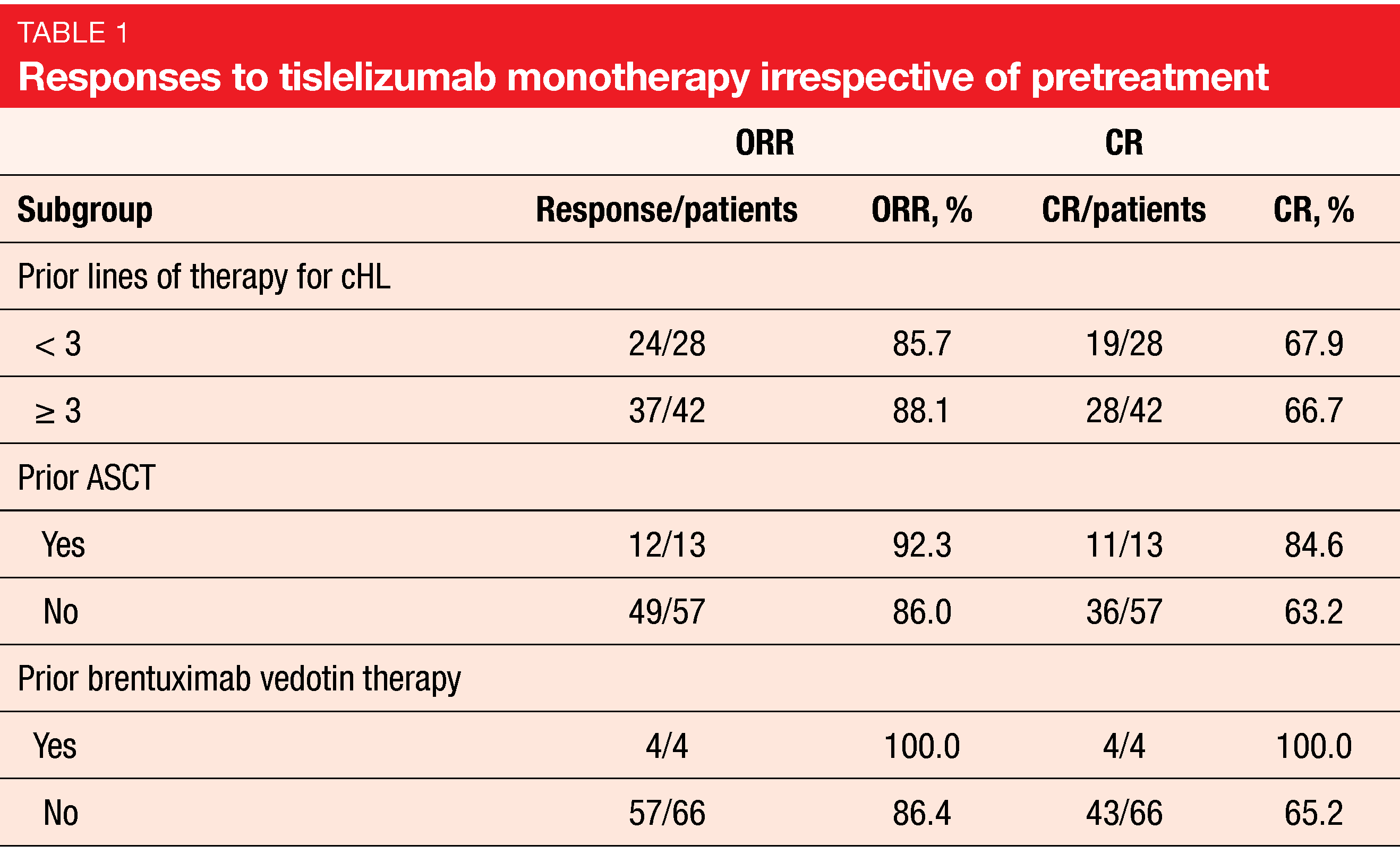
After a follow-up of 33.8 month, tislelizumab proved highly active. Objective responses occurred in 87.1 %, and CRs were achieved by 67.1 % of patients. Complete remissions emerged irrespective of the number of prior treatment lines and pretreatment with ASCT or BV (Table 1). The median time to response was 12 weeks, with 72 % of complete responders obtaining CR at the first post-baseline tumor assessment. Median PFS was 31.5 months. At the time of the 36-month landmark analysis, 40.8 % of patients remained progression-free. Patients who had achieved CR fared better in terms of PFS compared to those with PR and stable disease (not reached vs. 13.2 months). The OS results were immature; at 36 months, 84.8 % of patients lived, which is favorable considering the relapsed/refractory disease setting.
Grade ≥ 3 TRAEs occurred in 31.4 %, and in 8.6 %, AEs necessitated treatment discontinuation. irAEs were observed in 45.7 % and mainly included hypothyroidism (28.6 %), skin reactions (8.6 %), and pneumonitis (7.1 %). No new safety concerns were identified. In their summary, the authors noted that tislelizumab conferred a favorable benefit-risk profile and might represent an important treatment option for patients with relapsed or refractory cHL. A confirmatory phase III study is ongoing.
Update on camrelizumab
Camrelizumab has been approved in China based on the primary results of a multicenter, phase II trial in 75 patients with relapsed/refractory cHL in whom ASCT had failed to induce (lasting) remission or who had received ≥ 2 lines of systemic chemotherapies. The primary analysis yielded IRC-assessed objective responses in 76 % of patients, including CR in 28.0 % [14]. Follow-up data presented at EHA 2021 after 36.2 months demonstrated an ORR of 76.0 % by IRC assessment, with 26.7 % of patients experiencing CR [20]. Overall, 94.7 % achieved disease control. More than half showed ongoing responses at the time of the analysis. Median duration of response and median PFS were 31.7 and 22.5 months, respectively. Median OS had not been reached yet; at 36 months, 82.7 % of patients were alive.
The most common AE was cutaneous reactive capillary endothelial proliferation (RCEP) that occurred in almost all patients but was classified exclusively as grade 1 or 2. Complete resolution of RCEP lesions occurred in 67.1 %, with median time to complete resolution of 8.2 months. Most of the RCEP lesions regressed spontaneously, and none required systemic corticosteroids. At 12 and 24 months, the recurrence rates were 19.0 % and 6.3 %, respectively. No new toxicities emerged during the prolonged follow-up. AEs led to treatment interruption and discontinuation in 40.0 % and 6.7 %, respectively. The authors concluded that camrelizumab monotherapy continued to provide robust and durable responses as well as favorable survival and a manageable safety profile.
Promising results for penpulimab
Penpulimab is a novel IgG1 anti-PD-1 antibody engineered to eliminate binding to FcγRIa and FcγRIIIa receptors with the aim to avoid or reduce antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and antibody-dependent cytokine release. A multicenter, single-arm, open-label, phase I/II study investigated penpulimab 200 mg i. v. 2-weekly in patients with relapsed/refractory cHL following ASCT or ≥ 2 lines of chemotherapy. Updated phase II findings from the trial were presented at ASCO 2021 for 94 and 85 patients included in the safety and full analysis sets, respectively [25]. Almost 65 % of patients in the full analysis set had stage IV disease, and ≥ 3 prior lines of therapy had been administered in more than half of cases.
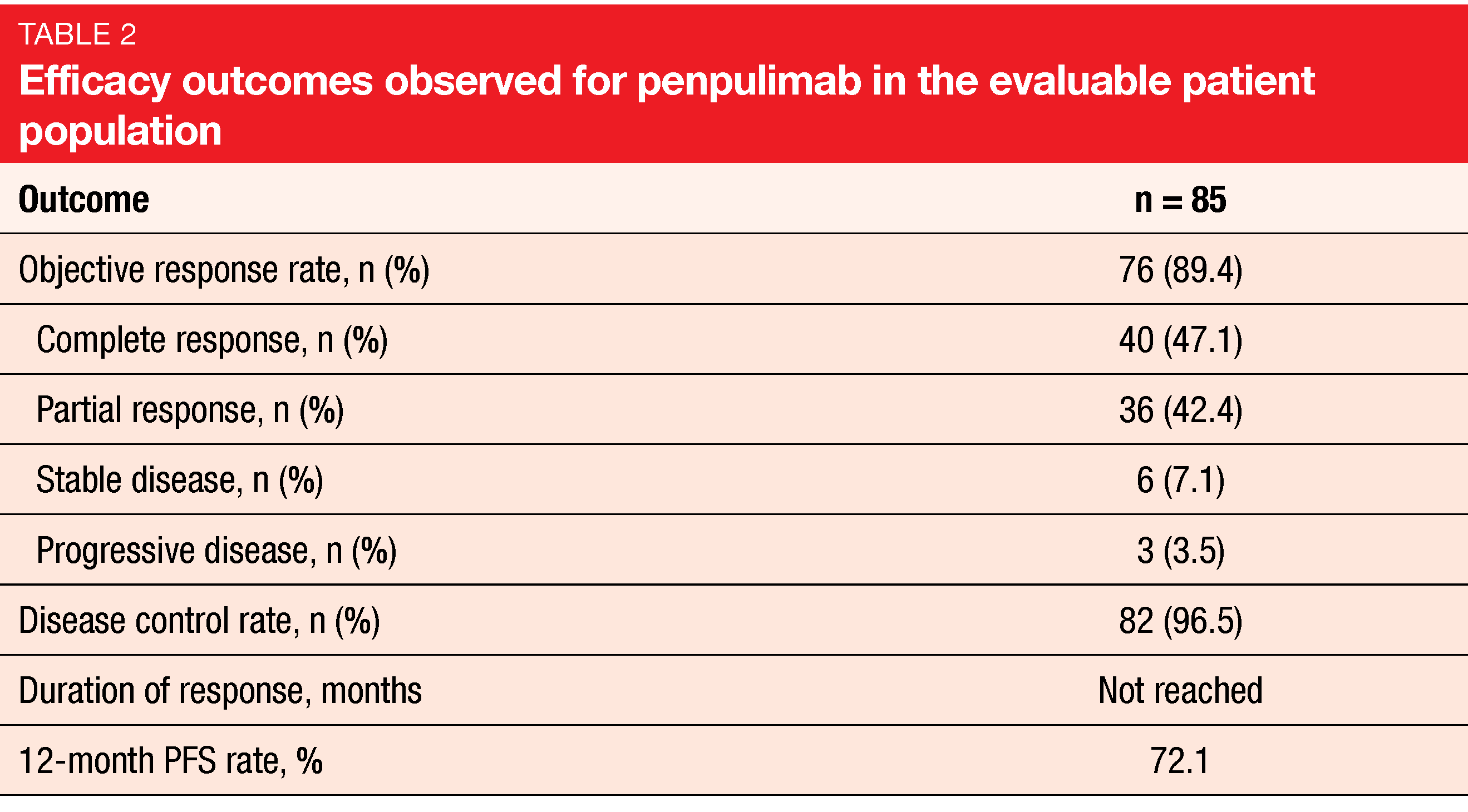
Among 85 evaluable patients, ORR with penpulimab was 89.4 %, and 47.1 % achieved CR (Table 2). The ORR benefit prevailed in all evaluated subgroups. Median duration of response had not been reached, which also applied to median PFS and median OS. All patients were alive at 18 months. The most frequent TRAEs included hypothyroidism (31.9 %), upper respiratory tract infection (25.5 %), fever (24.5 %) and alanine aminotransferase elevations (23.4 %). Grade 3 TRAEs were observed in 26.6 %, and treatment was discontinued in 5.3 %. Penpulimab showed a favorable immune-related safety profile. Grade 3 events occurred in 4.3 %, and no patient developed grade 4/5 events. Excluding thyroid disease, irAEs were reported in 17.0 % of the population.
Chidamide in addition to decitabine/camrelizumab
The combination of the anti-PD-1 antibody camrelizumab with the DNA-demethylating agent decitabine was evaluated in the clinical trial setting based on the observation that inhibition of de novo methylation can increase T-cell responses and tumor control during PD-1 inhibitor therapy in mice [21, 22]. Indeed, in a randomized phase II trial, camrelizumab plus decitabine elicited CRs in 79 % of patients with relapsed/refractory cHL, compared to 32 % with camrelizumab alone (p = 0.001) [23]. However, effective options have been lacking for patients who progressed on this regimen. A phase II study therefore tested a combined approach encompassing the histone deacetylase inhibitor chidamide in patients with relapsed/refractory cHL who had progressed or relapsed on camrelizumab/decitabine. Nineteen patients were treated with chidamide 10 mg (days 1–4) and 20 mg (days 8, 11,15, 18) plus decitabine 10 mg (days 1–5) and camrelizumab 200 mg (day 6) every 3 weeks.
According to preliminary results reported at ASCO 2021, the triple combination induced pronounced responses and showed an acceptable safety profile [24]. Among 14 patients who completed the response evaluation, 13 (93 %) experienced objective responses, including CRs in six cases (43 %). The most common AEs were leukopenia (58 %; grade 3, 16 %), nausea (53 %) and hypertriglyceridemia (26 %). No irAEs occurred.
REFERENCES
- Josting A et al., Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood 2000; 96(4): 1280-1286
- Ansell SM, Hodgkin lymphoma: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol 2012; 87(12): 1096-1103
- Bonfante V et al., Outcome of patients with Hodgkin’s disease failing after primary MOPP-ABVD. J Clin Oncol 1997; 15(2): 528-534
- Longo DL et al., Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin’s disease after combination chemotherapy: the low probability for cure. J Clin Oncol 1992; 10(2): 210-218
- Green MR et al., Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010; 116(17): 3268-3277
- Roemer MGM et al., PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 2016; 34(23): 2690-2697
- Carey CD et al., Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 2017; 130(22): 2420-2430
- Ansell SM et al., PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372(4): 311-319
- Chen R et al., Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017; 35(19): 2125-2132
- Kuruvilla J et al., Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2021; 22(4): 512-524
- Villasboas JC, Ansell SM, Nivolumab for the treatment of classical Hodgkin lymphoma after failure of autologous stem cell transplant and brentuximab. Expert Rev Anticancer Ther 2016; 16(1): 5-12
- Song Y et al., Treatment of relapsed or refractory classical Hodgkin lymphoma with the anti-PD-1, tislelizumab: results of a phase 2, single-arm, multicenter study. Leukemia 2020; 34(2): 533-542
- Shi Y et al., Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol 2019; 6(1): e12-e19
- Song Y et al., single-arm, multicenter, phase II study of camrelizumab in relapsed or refractory classical Hodgkin lymphoma. Clin Cancer Res 2019; 25(24): 7363-7369
- Armand P et al., Nivolumab for relapsed or refractory classical Hodgkin lymphoma after autologous hematopoietic cell transplantation: extended follow-up of the phase 2 single-arm CheckMate 205 study. Blood 2018; 132(suppl 1): 2897
- Ansell SM et al., Nivolumab for relapsed or refractory classical Hodgkin lymphoma after autologous transplantation: 5-year overall survival from the phase 2 CheckMate 205 study. ICML 2021, 074
- Dahan R et al., FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell 2015; 28(3): 285-295
- Arlauckas SP et al., In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med 2017; 9(389): eeal3604
- Song Y et al., Tislelizumab (BGB-A317) for relapsed/refractory classical Hodgkin lymphoma: long-term follow-up efficacy and safety results from a phase 2 study. EHA 2021, S207
- Wu J et al., Camrelizumab for relapsed or refractory classical Hodgkin lymphoma: three-year outcome of a multicenter, phase 2 study. EHA 2021, S206
- Ghoneim HE et al., De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 2017; 170(1): 142-157
- Pauken KE et al., Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016; 354(6316): 1160-1165
- Liu Y et al., Improved clinical outcome in a randomized phase II study of anti-PD-1 camrelizumab plus decitabine in relapsed/refractory Hodgkin lymphoma. J Immunother Cancer 2021; 9(4): e002347
- Wang C et al., Safety and efficacy of chidamide in combination with decitabine plus anti-PD-1 camrelizumab after relapse or progression on decitabine-plus-camrelizumab in classical Hodgkin lymphoma. J Clin Oncol 39, 2021 (suppl 15; abstr e19515)
- Song Y et al., A phase II study of penpulimab, an anti-PD-1 antibody, in patients with relapsed or refractory classic Hodgkin lymphoma (cHL). J Clin Oncol 39, 2021 (suppl 15; abstr 7529)
© 2021 Springer-Verlag GmbH, Impressum