Mantle cell lymphoma: improving outcomes in difficult-to-treat patient populations
Updated results from MAGNIFY
Mantle cell lymphoma (MCL) accounts for approximately 3–10 % of non-Hodgkin lymphomas and shows one of the poorest survival rates among the lymphomas [1, 2]. The combination of lenalidomide and rituximab (R2) has demonstrated activity in MCL patients in the frontline and relapsed/refractory settings, with overall response rates (ORRs) of 92 % and 57 %, respectively [3-5]. R2 is being explored in the multicenter, randomized, phase III MAGNIFY trial as initial and extended treatment in patients with relapsed/refractory NHL (i.e., follicular lymphoma grades 1-3b, transformed follicular lymphoma, marginal zone lymphoma, or MCL) after ≥ 1 prior therapy. Induction treatment consists of twelve 28-day cycles of lenalidomide 20 mg/d on day 1-21 and rituximab 375 mg/m2 weekly in cycle 1 followed by administration on day 1 of every other cycle. Subsequently, patients who have achieved complete or unconfirmed complete response (CR, CRu), partial remission, or disease stabilization, are randomized to either lenalidomide plus rituximab maintenance or rituximab maintenance for 18 cycles.
Progression-free survival (PFS) following randomization to R2 or rituximab monotherapy maintenance is the primary endpoint of the study. The first report in patients with relapsed/refractory MCL (n = 56) has revealed an ORR of 54 % [6]. Updated findings from the pre-randomization period of the study that assessed the combination only were presented at ICML 2021 by Sharman et al. [7].
Responses irrespective of rituximab sensitivity
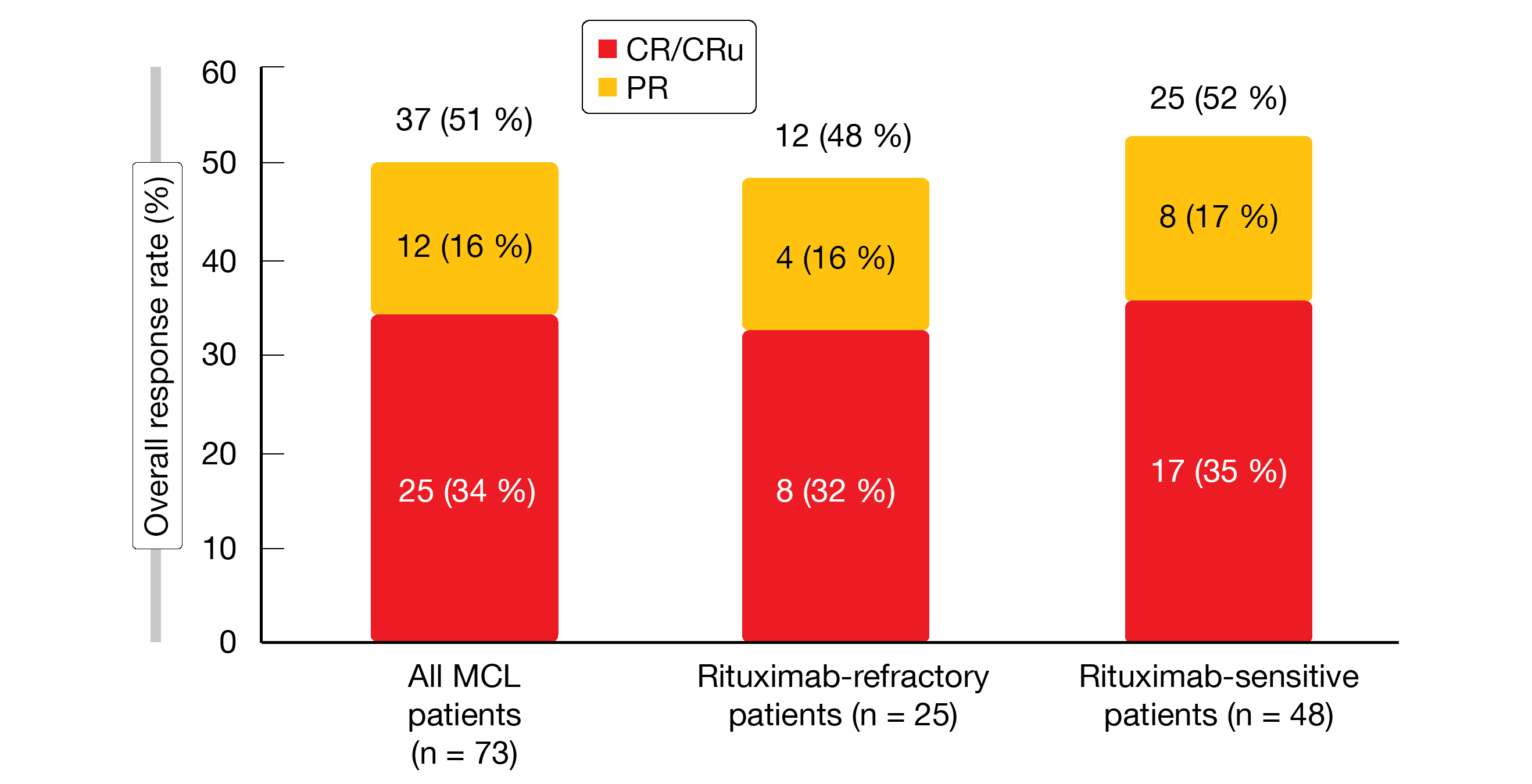
The MCL population included in this analysis comprised 73 patients who had received a median of two prior systemic therapies. All of them had been exposed to rituximab, and 34 % were rituximab-refractory. In the entire MCL group, initial treatment elicited an ORR of 51 %, with 34 % of patients achieving CR/CRu. These findings were similar across the rituximab-refractory and rituximab-sensitive groups (Figure 1). Median time to response was 2.8 months, and the majority of patients experienced tumor size reductions of > 50 %. In the cohort that obtained CR or PR, the median duration of response was 31.6 months; for rituximab-refractory and rituximab-sensitive patients, this was 31.6 and 28.4 months, respectively. Median duration of complete response had not been reached in any subgroup. After a median follow-up of 31.7 months, the total group showed a median PFS of 28.0 months (14.7 and 30.1 months for rituximab-refractory and rituximab-sensitive patients, respectively).
The safety profile observed in the MCL cohort was consistent with previous reports of the overall MAGFNIFY population. Neutropenia emerged most frequently (51 %), with 46 % of patients experiencing grade 3/4 events, followed by fatigue (44 %), diarrhea (32 %), constipation (28 %), and cough (28 %). Although neutropenia was common, grade 3/4 febrile neutropenia occurred only in seven patients (10 %). In their conclusions, the authors noted that R2 is an active and tolerated regimen with durable responses among patients with relapsed/refractory MCL. The trial is ongoing to compare R2 with rituximab extended treatment.
Figure 1:Responses to lenalidomide/rituximab in the initial treatment period of the MAGNIFY study: total population and subgroups according to rituximab sensitivity
Venetoclax, lenalidomide & rituximab
Based on observations suggesting efficacy of venetoclax [8] and lenalidomide plus rituximab [9] in MCL patients, as well as synergistic effects of venetoclax plus lenalidomide [10], it was hypothesized that the chemotherapy-free combination of venetoclax, lenalidomide and rituximab would be safe with the potential to improve outcomes and induce minimal residual disease (MRD) negativity in this setting. At ICML 2021, Phillips et al. presented the results of a study that evaluated the triple regimen in patients with untreated MCL [11]. The induction phase included twelve cycles of lenalidomide 20 mg/d on day 1-21 every 28 days, venetoclax 400 mg/d after ramp-up, and rituximab 375 mg/m2 weekly in cycle 1 and every other month thereafter. Response assessment was performed during and after induction using PET/CT scan and MRD testing in peripheral blood. Patients who achieved radiographic CR and MRD negativity, as well as those without disease progression who were not eligible for transplant or declined it, went on to receive maintenance. Here, the lenalidomide dose was reduced by half of the induction dose and continued for 24 months, while venetoclax was continued for 12 months and rituximab was given every other cycle for 36 months.
Activity in high-risk patients
Overall, 28 patients were treated, with 19 (68 %) completing induction and transitioning to maintenance. At the time of the analysis, 23 patients (82 %) remained on treatment. Three and two patients, respectively, left the study due to progressive disease or other medical conditions. This was a poor-prognosis population; high risk according to the MIPI score was present in 88 %, and 14 % and 7 % of patients had the blastoid/blastic and pleomorphic variants, respectively.
Nevertheless, these early preliminary data indicated efficacy of the regimen. Radiographic assessment revealed CR in 67 % of 28 patients after 3 months and in all patients (n = 12) at 12 months. Likewise, the percentage for undetectable MRD rose from 63 % at 3 months (n = 27) to 92 % at 12 months (n = 12). The 12-month PFS rate was 86 %. No patient who had obtained undetectable MRD had relapsed until cutoff. Three patients experienced disease progression, with all of them harboring p53 mutations.
Dose reductions or delays were necessary in 53 %. No dose-limiting toxicities occurred, and all patients were able to safely escalate venetoclax to 400 mg/d. The most common AEs included neutropenia, thrombocytopenia, diarrhea, and anemia, which were also the most common grade 3/4 AEs. Two secondary cancers (i.e., adenocarcinoma of unknown primary, cutaneous squamous cell carcinoma) emerged on study, and two disease-related deaths occurred. Future plans include the expansion of the original study to enroll another 50 patients who do not harbor the p53 mutation. However, this patient group will also be addressed as modification of the regimen to specifically target the p53 mutation and other high-risk features is planned.
Real-world data on rituximab maintenance
Randomized clinical studies have yielded improved survival with rituximab maintenance (MR) following first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in both young and older MCL patients [12, 13]. Guidelines therefore recommend MR after first-line treatment [14, 15]. To date, however, real-world evidence regarding the use of MR in routine clinical practice has been limited. In particular, the benefits of MR after first-line bendamustine plus rituximab (BR) are not well established. At EHA 2021, Salles et al. reported a retrospective analysis evaluating real-world patterns and outcomes of MR after first-line BR or R-CHOP in a large US MCL cohort using the nationwide Flatiron Health database [16].
The MR-eligible cohort included 1,621 patients; by definition, they had received first-line induction combined with rituximab, and rituximab had been continued as monotherapy for ≥ 28 days. This population was subdivided into four groups: those treated with BR only (n = 728; 44.9 %), BR plus MR (n = 458; 28.3 %), R-CHOP only (n = 255; 15.7 %), and R-CHOP plus MR (n = 180; 11.1 %). To date, this is the largest real-world cohort of patients with MCL treated mostly in community-based US practices.
MR as independent predictor
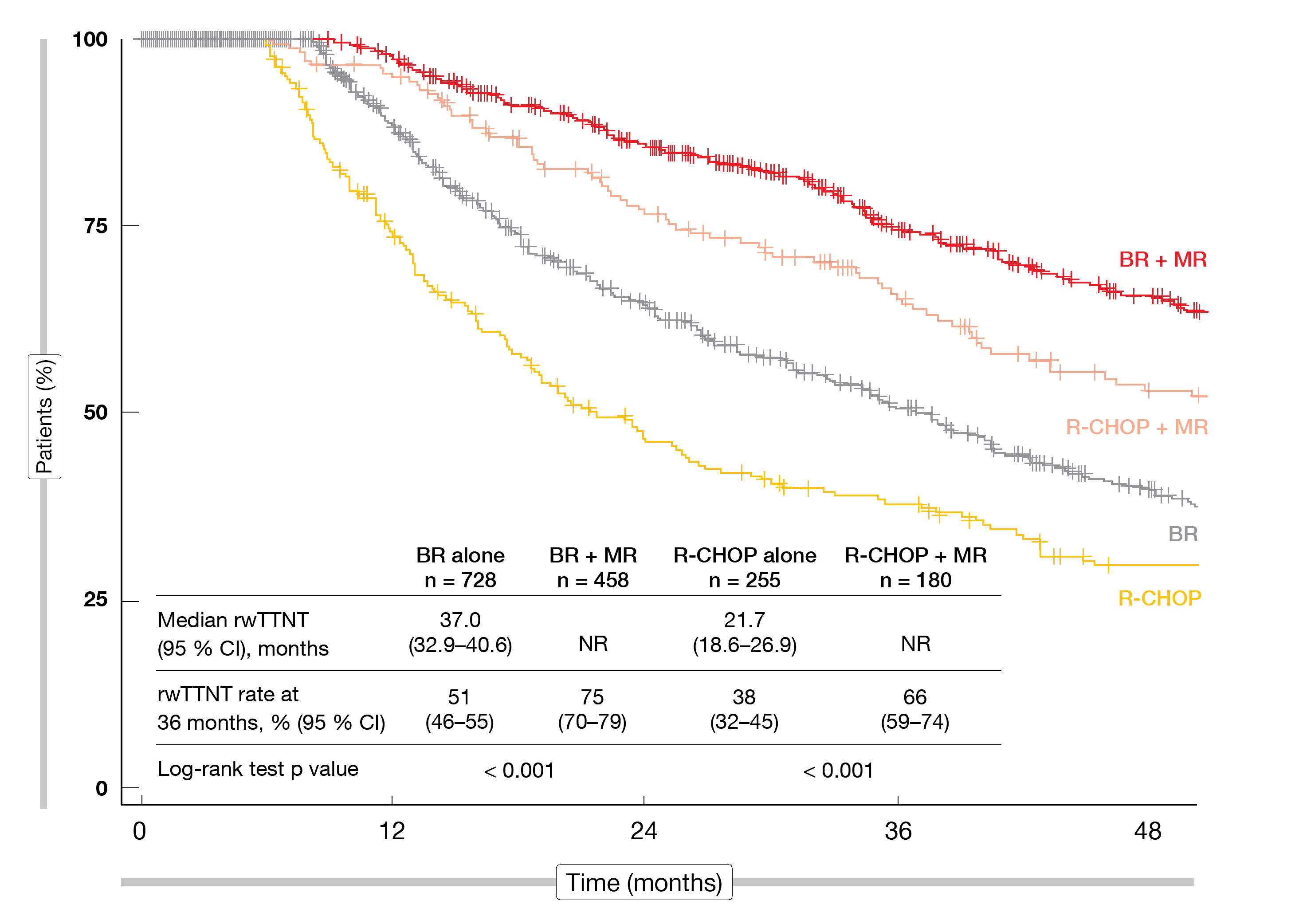
The use of MR in addition to both BR and R-CHOP conferred significantly improved outcomes. Median real-world time to next treatment was 37.0 months and 21.7 months, respectively, with BR and R-CHOP alone, while this had not been reached for either BR + MR or R-CHOP + MR (Figure 2). These differences were significant (p < 0.001 for both BR vs. BR + MR and R-CHOP vs. R-CHOP + MR). BR + MR performed relatively better than R-CHOP + MR. The real-world OS rates at 36 months amounted to 76 % vs. 85 % for BR vs. BR + MR (p = 0.001), and to 77 % vs. 88 % for R-CHOP vs. R-CHOP + MR (p = 0.030).
According to multivariate analyses, MR was an independent predictor of superior real-world time to next treatment and OS. Patient and disease characteristics associated with significantly worse outcomes included age ≥ 65 years, LDH/ULN ≥ 1, bulky disease, and blastoid/pleomorphic morphology. The authors pointed out that these results need to be interpreted in the context of evidence provided by randomized studies assessing MR in MCL as selection biases are inherent in real-world analyses. At present, the phase III SHINE trial is evaluating whether PFS can be improved with the addition of ibrutinib to BR followed by MR in older patients with previously untreated MCL.
Figure 2: Real-world time to next treatment (rwTTNT) for bendamustine/rituximab (BR) ± maintenance rituximab (MR) and R-CHOP ± maintenance rituximab
REFERENCES
- Parrott M et al., A systematic review of treatments of relapsed/refractory mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 2018(1); 18: 13-25
- Teras LR et al., 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016; 66(6): 443-459
- Ruan J et al., Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N Engl J Med 2015; 373(19): 1835-1844
- Ruan J et al., Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood 2018; 132(19): 2016-2015
- Wang M et al., Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 2012; 13(7): 718-723
- Sharman J et al., MAGNIFY phase IIIB interim analysis: first report of induction R2 followed by maintenance in patients with relapsed/refractory mantle cell lymphoma. Hematol Oncol 2019; 37(S2): 241-242
- Sharman JP et al., Induction R2 followed by maintenance in patients with relapsed/refractory mantle cell lymphoma: interim analysis from the phase 3b MAGNIFY study. ICML, 063
- Davids MS et al., Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. Clin Oncol 2017; 35(8): 826-833
- Yamshon S et al., Initial treatment with lenalidomide plus rituximab for mantle cell lymphoma: 7-year analysis from a multicenter phase III study. ASH 2020, 704
- Paulus A et al., AT-101 downregulates BCL2 and MCL1 and potentiates the cytotoxic effects of lenalidomide and dexamethasone in preclinical models of multiple myeloma and Waldenström macroglobulinaemia. Br J Haematol 2014; 164(3): 352-365
- Phillips TJ et al., The combination of venetoclax, lenalidomide and rituximab in patients with newly diagnosed mantle cell lymphoma induces high response rates and MRD undetectability. ICML 2021, 061
- Kluin-Nelemans HC et al., Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012; 367(6): 520-531
- Le Gouill S et al., Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med 2017; 377(13): 1250-1260
- NCCN Clinical Practice Guidelines in Oncology B-Cell Lymphoma, version 4-2021-February 16, 2021
- McKay P et al., Guideline for the management of mantle cell lymphoma. Br J Haematol 2018; 182(1): 46-62
- Salles G et al., Role of maintenance rituximab after first-line bendamustine + rituximab or R-CHOP in patients with mantle cell lymphoma form a large US real-world cohort. EHA 2021, EP785
© 2021 Springer-Verlag GmbH, Impressum