Successful inhibition of PI3K, BTK, BCL2 and other targets in various B-cell malignancies
CHRONOS-3: copanlisib plus rituximab
Rituximab monotherapy is a recognized standard of care in patients with relapsed indolent non-Hodgkin lymphoma (iNHL) who have had long remissions after rituximab-based therapy or who are unwilling or unfit to be treated with chemotherapy. However, the clinical benefit conferred by rituximab can be limited due to drug resistance. Based on the assumption that a combined strategy might improve patient outcomes, the phase III CHRONOS-3 trial investigated the combination of rituximab with the selective, potent, pan-class I PI3K inhibitor copanlisib that shows predominant on-target activity against the PI3K-α and PI3K-δ isoforms [1, 2].
CHRONOS-3 recruited patients with relapsed CD20-positive indolent B-cell lymphoma including follicular lymphoma (FL) grades 1-3a, marginal zone lymphoma (MZL), small lymphocytic lymphoma (SLL), and Waldenström’s macroglobulinemia (WM). Their disease had relapsed following regimens containing rituximab or anti-CD20 antibodies. Eligible patients had to be progression- and treatment-free for ≥ 12 months since the last rituximab-containing regimen or for ≥ 6 months and unwilling or unfit to receive chemotherapy. The study treatment consisted of copanlisib 60 mg i. v. on days 1, 8 and 15 of a 28-day cycle plus rituximab (n = 307), or placebo plus rituximab (n = 151). Median time since the last systemic therapy was 25.1 months in both arms; approximately half of patients had received ≥ 2 prior lines of anti-cancer treatment. Progression-free survival (PFS) by central review was defined as the primary endpoint. The primary analysis of the study was reported at EHA 2021 by Zinzani et al. [3].
Benefits across all subtypes
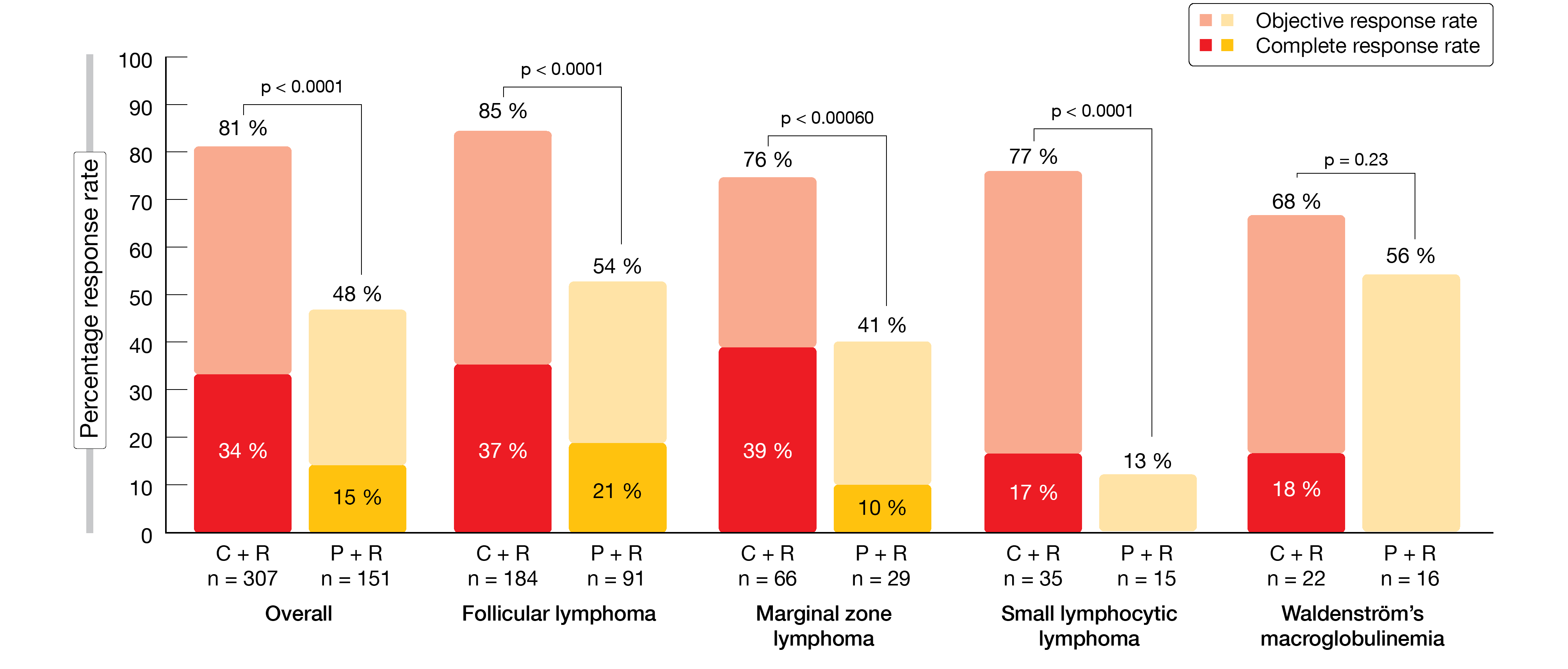
CHRONOS-3 met its primary endpoint. Compared to rituximab alone, copanlisib/rituximab induced a 48 % reduction in the risk of progression or death, with a median PFS of 21.5 vs. 13.8 months (HR, 0.52; p < 0.0001). PFS benefits were observed across all iNHL subtypes and in all pre-specified subgroups pertaining to patient and disease characteristics. In the total study population, objective responses occurred in 81 % vs. 48 % (p < 0.0001; Figure 1). Complete responses (CRs) resulted in 34 % vs. 15 %. Objective response rates (ORRs) and CR rates were improved in the experimental arm compared to the control arm across all histologic subtypes. Reductions in target lesion size were observed in 98 % of evaluable patients with copanlisib/rituximab and in 86 % with placebo/rituximab. The trend towards greater tumor shrinkage in the experimental arm was consistent across histologic subtypes.
Patient-reported outcomes constituted a secondary endpoint. These were reported as the time to deterioration or improvement in ≥ 3 points in patient-reported disease-related physical symptoms according to the FLymSI-18 questionnaire. Here, the analysis revealed no significant differences across the two treatment arms regarding time to deterioration (5.5 months each; HR, 1.060; p = 0.69) and time to improvement (not estimable in either arm; HR, 0.996; p = 0.51).
Copanlisib plus rituximab demonstrated a manageable safety profile that was consistent with previous reports of both agents as monotherapies. Hyperglycemia was the most frequently reported treatment-emergent adverse event (AE) in both arms (69.4 % vs. 23.3 %), followed by hypertension (49.2 % vs. 19.2 %). Copanlisib-related hyperglycemia and hypertension proved transient and manageable. Pneumonitis occurred in 6.8 % vs. 1.4 % (grade 3, 2.0 % vs. 0.7 %). In their conclusions, the authors pointed out that the addition of copanlisib to standard rituximab treatment demonstrated broad and superior efficacy compared to rituximab monotherapy in patients with relapsed iNHL of all histologies. The combination represents a potential new treatment option for patients with relapsed iNHL of all subtypes.
Figure 1: CHRONOS-3: objective and complete responses observed for copanlisib plus rituximab (C + R) vs. placebo plus rituximab (P + R)
Zandelisib plus zanubrutinib
Dual inhibition of the PI3Kδ and BTK pathways is considered capable of overcoming monotherapy resistance, although tolerability can be an issue. Therefore, a multicohort phase 1b study explored the combination of the PI3Kδ inhibitor zandelisib, which offers an innovative dose schedule based on optimized pharmaceutical properties, and the BTK inhibitor zanubrutinib to improve tolerability of the dual targeted approach and to provide deeper and longer responses.
Patients after failure of ≥ 1 prior therapy for B-cell malignancies were treated until progression with one of two dose schedules. Group A received zandelisib 60 mg/d for 8 weeks followed by days 1–7 of each subsequent 28-day cycle, plus zanubrutinib 160 mg twice daily (n = 7). In Group B, zandelisib 60 mg was taken daily on days 1–7 of each 28-day cycle starting in cycle 1 in addition to zanubrutinib 80 mg twice daily (n = 13). At EHA 2021, Soumerai et al. reported on 20 patients in the completed safety dose-finding cohort [4].
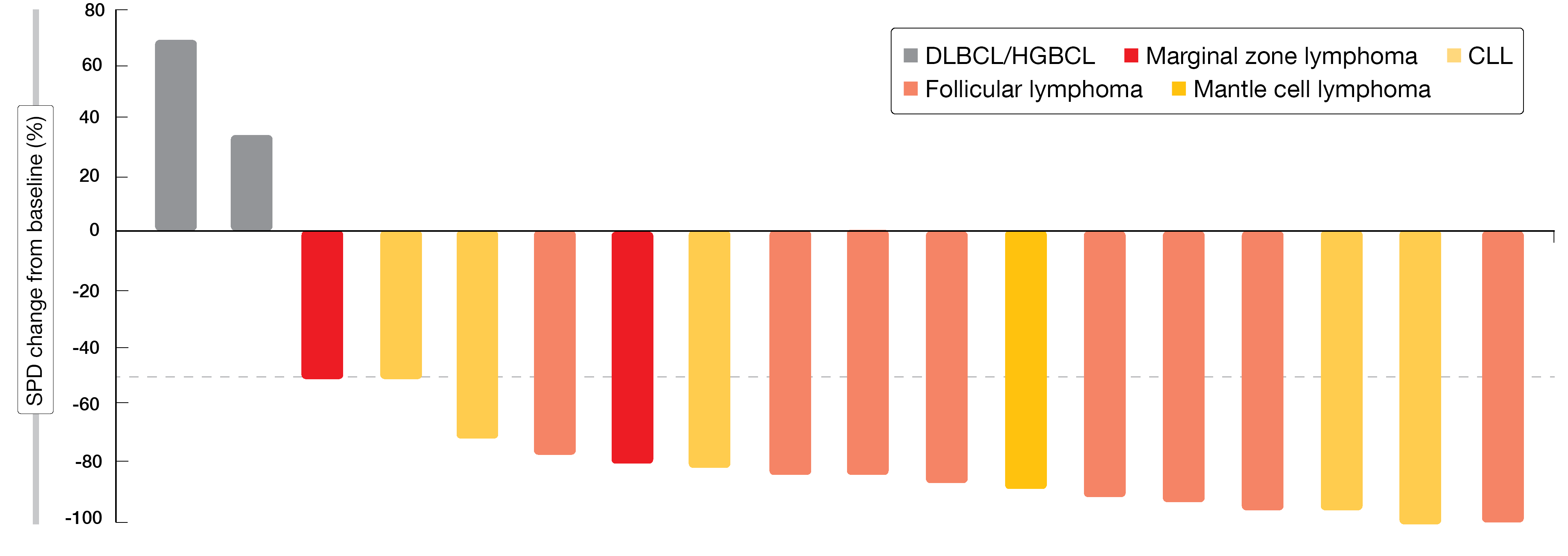
Disease-specific expansion cohorts were created (FL, CLL/SLL, MZL, mantle cell lymphoma [MCL], diffuse large B-cell lymphoma [DLBCL]/high-grade B-cell lymphoma [HGBCL]) that received the Group B dose schedule. Across these cohorts, 18 individuals were evaluable. All of them responded to zandelisib 60 mg on an intermittent schedule from cycle 1 plus zanubrutinib 80 mg twice daily except for two patients with DLBCL/HGBCL (Figure 2). Complete remissions with and without incomplete bone marrow recovery occurred in 25 % and 40 % of FL and CLL patients, respectively. The combination was generally well tolerated and did not result in additive toxicity compared to each agent alone. Neutropenia, thrombocytopenia, transaminase elevations, diarrhea, rash, hyperkalemia and fatigue represented the most common AEs. Patients are currently enrolled in expansion cohorts for FL and MCL with the regimen identified in Group B.
Another zandelisib-based combination is currently being tested in the setting of relapsed iNHL by the randomized, open-label, controlled, multicenter phase III COASTAL study [5]. Here, patients with FL or MZL after ≥ 1 prior line of therapy are receiving either zandelisib plus rituximab or standard immunochemotherapy. Pretreatment must include an anti-CD20 antibody in combination with chemotherapy or lenalidomide. Zandelisib is administered for two years, while rituximab or immunochemotherapy are given for a total of six cycles. The primary endpoint is PFS. Approximately 534 patients will be enrolled at 200 sites globally.
Figure 2: Maximum change of tumor diameter from baseline with zandelisib plus zanubrutinib
Zanubrutinib in ibrutinib/acalabrutinib intolerance
BTK inhibitor therapy is effective in B-cell malignancies, although its use is limited by AEs [6, 7]. The next-generation BTK inhibitor zanubrutinib, which has been optimized for BTK selectivity and occupancy, has shown increased tolerability compared to ibrutinib in patients with WM treated in the ASPEN trial [8]. Lower rates of AEs leading to death (1 % vs. 4.1 %), discontinuation (4 % vs. 9.2 %), dose reductions (13.9 % vs. 23.5 %) and dose holds (46.5 % vs. 56.1 %) were observed, as well as a lower rate of atrial fibrillation or flutter (2 % vs. 15.3 %).
The multicenter, single-arm, open-label phase II BGB-3111-215 study investigated the safety and efficacy of zanubrutinib in patients with pretreated B-cell malignancies who were intolerant to ibrutinib and/or acalabrutinib, compared with their ibrutinib and/or acalabrutinib intolerance as assessed by the recurrence and change in severity of AEs. Patients with previously treated CLL/SLL, WM, MCL, or MZL were divided in those intolerant to ibrutinib (Cohort 1) and those intolerant to acalabrutinib alone or to both agents (Cohort 2). They were treated with zanubrutinib 160 mg twice daily or 320 mg per day.
The analysis reported at EHA 2021 related to 57 and 7 patients included in Cohort 1 and 2, respectively [9]. Most of them had received prior ibrutinib monotherapy. The median time on the most recent prior BTK inhibitor was 9.2 months in the total cohort. Overall, 75 % of ibrutinib and acalabrutinib intolerance events did not recur on zanubrutinib. Among the intolerance events that recurred, 90 % and 33 % of ibrutinib and acalabrutinib intolerance events, respectively, recurred at a lower severity, while 10 % and 67 %, respectively, occurred at the same severity. No intolerance events recurred at a higher grade.
Zanubrutinib itself proved effective, with ORR and disease control rates of 50.0 % and 89.6 %, respectively, in the total population. At data cutoff, 87.7 % and 100 % of patients in Cohorts 1 and 2, respectively, remained on treatment. AEs were the reason for zanubrutinib treatment discontinuation in three cases; none of these were due to recurrence of a prior intolerance event. The authors emphasized that zanubrutinib might provide a therapeutic option in patients intolerant to other BTK inhibitors across hematologic malignancies.
Broad efficacy of pirtobrutinib
Non-covalent BTK inhibition might offer advantages over covalent inhibitors that have been demonstrated to elicit limited OS benefits in patients with MCL and other lymphomas [10, 11]. The highly potent and selective non-covalent BTK inhibitor pirtobrutinib (LOXO-305) is being assessed in the phase I/II BRUIN trial in patients with previously treated advanced B-cell malignancies who have received all classes of available therapy.
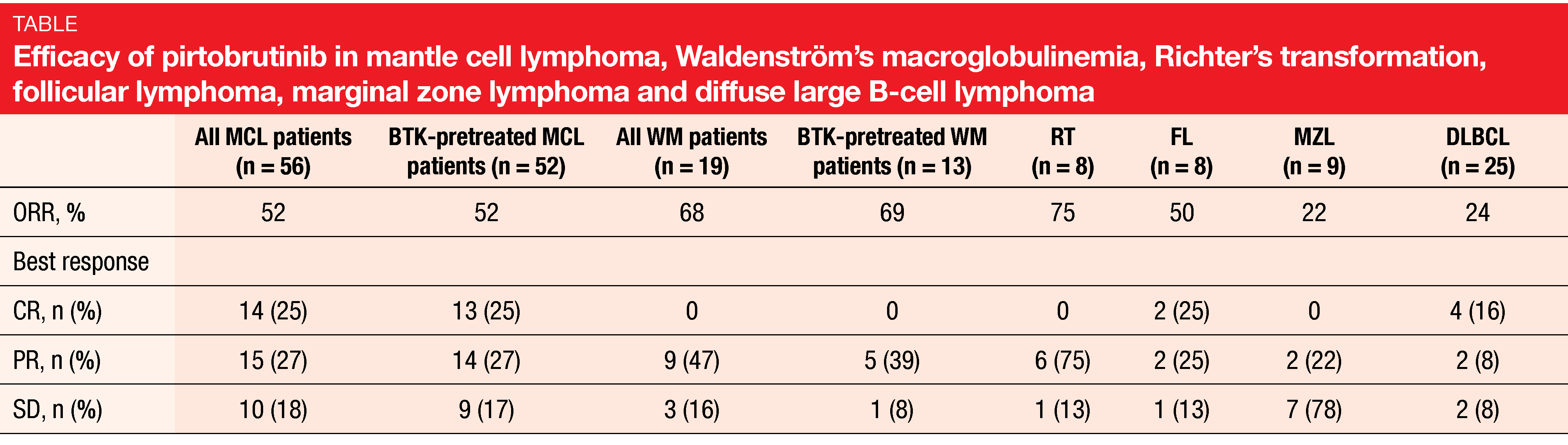
According to the analysis presented at EHA 2021, pirtobrutinib showed promising efficacy with responses across all dose levels and clinical activity independent of prior therapy [12]. MCL patients (n = 56) demonstrated a 52 % ORR, with CRs emerging in 25 %, which also applied to BTK-pretreated MCL patients (n = 52; Table). Those with other NHLs showed response rates ranging from 22 % (MZL) to 75 % (Richter’s transformation; Table). After a median follow-up of 6 months for the efficacy-evaluable population, 83 % of responding MCL patients were ongoing and in response. Given the poor outcomes with existing options for the treatment of MCL, these results are particularly notable. Also, 77 % and 83 % of responders in the groups with WM and Richter’s transformation were ongoing and in response.
The favorable safety and tolerability were consistent with the design of pirtobrutinib as a highly selective and non-covalent BTK inhibitor. No dose-limiting toxicities occurred, and the maximum tolerated dose was not reached. Pirtobrutinib 200 mg/d was selected as the recommended phase II dose. Only 1.5 % of patients discontinued treatment due to treatment-related AEs. Notable covalent BTK-inhibitor–associated toxicities were rarely observed. The authors concluded that pirtobrutinib is well tolerated and exhibits promising efficacy in heavily pretreated patients with MCL and other NHLs. Longer follow-up is required to better understand the safety profile associated with chronic administration.
TG-1701 ± U2
An emerging BTK inhibitor with favorable characteristics is the covalently bound agent TG-1701 that has shown superior selectivity compared to ibrutinib [13]. Data have indicated increased inhibition of TG-1701 in combination with the PI3Kδ inhibitor umbralisib and the anti-CD20 antibody ublituximab (U2) in a BTK-resistant cell model [14]. A phase I/II dose escalation trial presented at ASCO 2021 evaluated TG-1701 as monotherapy and combined with U2 in dose escalation cohorts and disease-specific cohorts [15]. The patients included had relapsed/refractory disease to prior standard therapy; in addition, the disease-specific cohorts enrolled treatment-naïve patients who were unsuitable for standard front-line chemoimmunotherapy. TG-1701 doses were escalated across a range of 100-400 mg/d as monotherapy and 100-300 mg/d as part of the combination regimen.
TG-1701 exhibited an encouraging safety profile with clinical and pharmacodynamic activity at all dose levels that supported once-daily dosing. The maximum tolerated dose was not achieved in the monotherapy arm. Respiratory tract infections, constipation, bruising, neutropenia and ALT elevations were seen most frequently, with the majority of AEs rated as grade 1/2. Patients on the triplet regimen developed most commonly diarrhea, infusion-related reactions, and bruising (47 % each). Among grade 3 laboratory AEs, ALT and AST prevailed (16 % each). Dose escalation continues.
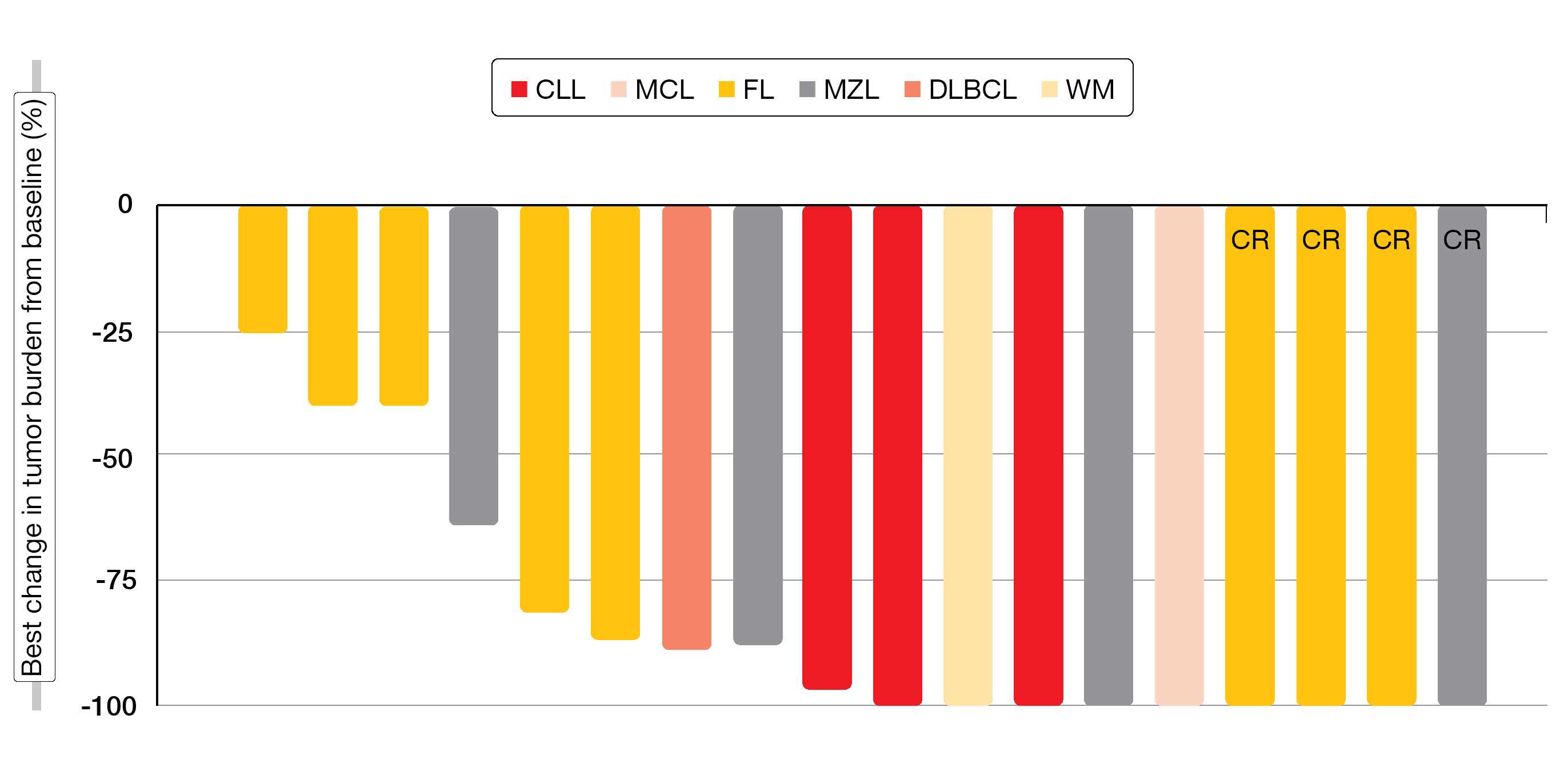
Single-agent TG-1701 100–400 mg gave rise to an ORR of 57 % across a range of histologic subtypes including CLL, SLL, MCL, FL, MZL, and WM, while patients with DLBCL did not respond. In the disease-specific cohorts, the ORRs with TG-1701 monotherapy at a dose of 200 mg were 95 %, 65 % and 95 % for CLL, MCL, and WM, respectively. All patients treated in the CLL cohort with TG-1701 300 mg achieved objective responses. For the triplet combination, the ORR was 79 %, with a CR rate of 21 %, across all subtypes (Figure 3). After a median follow-up of 15.6 months, most patients remained on treatment. The study continues enrollment, and future registration trials are being planned.
Figure 3: Changes in tumor burden observed with TG-1701 (dose range, 100-300 mg/d) plus umbralisib and ublituximab
Atezolizumab-based triple combination
Combining targeted therapies with agents that enhance anti-tumor immunity represents an attractive treatment paradigm. A multicenter phase II trial evaluated the PD-L1 inhibitor atezolizumab in combination with the anti-CD20 antibody obinutuzumab and the BCL2 inhibitor venetoclax in patients with relapsed/refractory FL and MZL who had failed ≥ 1 line of therapy. Induction included eight 3-weekly cycles of obinutuzumab. Venetoclax was started on day 8 of cycle 1 and was administered until the end of maintenance or until progression. Atezolizumab was given on day 2 of each cycle. ORR at the end of induction after eight cycles of the three drugs or at premature treatment discontinuation constituted the primary endpoint.
Herbaux et al. presented the results for the FL and MZL cohorts at EHA 2021 [16]. The FL cohort comprised 58 patients, 85.7 % of whom had Ann Arbor stage III/IV disease. High risk according to the Follicular Lymphoma International Prognostic Index was present in 47.3 %. One third of patients had been treated with > 2 lines of previous therapy, and 30.4 % had undergone autologous stem cell transplantation. In this group, 63 % of patients received the full induction treatment. The ORR according to PET scan at the end of induction was 53.6 %, with complete molecular remissions in 30.4 %.
In the MZL cohort, 20 patients were enrolled. Thirteen, five and two had nodular, extra-nodular and splenic MZL, respectively. All of them were in Ann Arbor stage IV, 38.9 % showed bone marrow infiltration, and 50 % had ≥ 2 extra-nodal sites. In 22.2 %, > 2 prior lines of treatment had been administered. Eleven patients (55 %) received the full induction treatment. According to CT assessment at the end of induction, 66.76 % of patients demonstrated objective responses, which included complete and partial remissions in 16.7 % and 50.0 %, respectively. At the time of the analysis, responses in the two cohorts appeared to be durable, with only 21.4 % of responders experiencing relapses or progression.
In 70.5 % of the total study cohort, grade 3/4 AEs occurred, and one patient experienced an AE that led to discontinuation of treatment. Grade 3/4 AEs were mainly cytopenias, with only one case of febrile neutropenia (1.3 %). Three patients developed immune-related AEs classified as grade 2 or 3. No tumor lysis syndrome occurred. Overall, the combination appeared to be well tolerated, with no unexpected toxicity. The authors concluded that the ORR results were comparable to the findings seen for other innovative regimens in this setting.
Novel BCL2 inhibitor BGB-11417
Cheah et al. provided the initial clinical report of the first-in-human trial investigating the potent and highly selective BCL2 inhibitor BGB-11417 at EHA 2021 [17]. In vitro evaluation has shown that BGB-11416 is > 10-fold more potent than venetoclax at inhibiting BCL2 (IC50, 0.014 vs. 0.20 nM) [18]. Moreover, the potency of BGB-11417 for inhibiting the BCL2-G101V mutant protein, which can induce resistance to venetoclax on continued treatment, was > 50-fold higher than that of venetoclax. BGB-11417 demonstrated ≥ 2,000-fold selectivity for BCL2 compared to BCL-xL, BCL-W, MCL-1, and BCL2A1 in vitro, which illustrates its selectivity. Various xenograft models including acute lymphoblastic leukemia, MCL and DLBCL revealed superior anti-tumor activity of BGB-11417 vs. venetoclax. The novel BCL2 inhibitor exhibits a favorable pharmacokinetic profile with low plasma clearance and an encouraging safety profile [19].
The first-in-human phase I/Ib study is being conducted in patients with relapsed and refractory B-cell malignancies including NHL (i.e., FL, DLBCL, MZL, transformed NHL), CLL/SLL, MCL, and WM. Dose escalation (Part 1) is performed in independent cohorts categorized by disease type. These cohorts will continue until a recommended phase II dose is identified, which is then used in corresponding expansion cohorts (Part 2). The study will also include dose escalation and expansion cohorts for the combination of BGB-11417 and the BTK inhibitor zanubrutinib in patients with CLL/SLL and mantle cell lymphoma.
At the time of the first analysis, seven patients with relapsed/refractory NHL were treated in Cohort 1A, and two patients with relapsed/refractory CLL were treated in Cohort 1B. According to the results, BGB-11417 is tolerable at the dose levels tested, with no dose-limiting toxicities observed across two dose levels. Grade ≥ 3 AEs were infrequent and manageable. Two patients experienced neutropenia, and only one instance of laboratory TLS was recorded in a patient with high TLS risk. Preliminary activity will be assessed with increased enrollment and follow-up. Although enrollment of patients with relapsed/refractory CLL has only recently started, decreases in absolute lymphocyte counts were already seen at the initial ramp-up dose of 1 mg.
Anti-CD19-based approach: ongoing study
The anti-CD19 antibody tafasitamab has been licensed by the US FDA for use in the indication of relapsed/refractory DLBCL. In patients with relapsed/refractory FL or MZL, tafasitamab plus lenalidomide/rituximab is being evaluated versus lenalidomide/rituximab alone in the randomized, double-blind, placebo-controlled, phase III inMIND study [20]. PFS in the FL cohort has been defined as the primary endpoint of the trial. At present, recruitment is ongoing in centers across Europe, Asia Pacific and North America. The planned enrollment comprises 528 patients with relapsed/refractory FL and 60–90 patients with relapsed/refractory MZL.
REFERENCES
- Liu N et al., BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther 2013; 12(11): 2319-2330
- Morschhauser F et al., On-Target Pharmacodynamic Activity of the PI3K Inhibitor Copanlisib in Paired Biopsies from Patients with Malignant Lymphoma and Advanced Solid Tumors. Mol Cancer Ther 2020; 19(2): 468-478
- Zinzani P et al., CHRONOS-3: randomized phase III study of copanlisib plus rituximab vs rituximab/placebo in relapsed indolent non-Hodgkin lymphoma. EHA 2021, S211
- Soumerai JD et al., Combination of PI3Kδ inhibitor zandelisib and the BTK inhibitor zanubrutinib in patients with relapsed or refractory B-cell malignancies. EHA 2021, S214
- Jurczak W et al., COASTAL: A phase 3 study of the PI3KΔ inhibitor zandelisib with rituximab versus immunochemotherapy in patients with relapsed indolent non-Hodgkin’s lymphoma. EHA 2021, PB1569
- Mato AR et al., Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica 2018; 103(5): 874-879
- Yazdy MS et al., Toxicities and outcomes of acalabrutinib-treated patients with chronic lymphocytic leukemia: a retrospective analysis of real world patients. Blood 2019; 134(Suppl 1, abstr 4311)
- Dimopoulos MA et al., ASPEN: results of a phase 3 randomized trial of zanubrutinib versus ibrutinib for patients with Waldenström macroglobulinemia. EHA 2020, S225
- Shadman M et al., Preliminary results of a phase 2 study of zanubrutinib in patients with previously treated B cell malignancies intolerant to ibrutinib/acalabrutinib. EHA 2021, EP642
- Cheah CY et al., Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol 2015; 26(6): 1175-1179
- Martin P et al., Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016; 127(12): 1559-1563
- Shah NN et al., Pirtobrutinib (LOXO-305), a next generation highly selective non-covalent Bruton’s tyrosine kinase inhibitor in previously treated mantle cell lymphoma and other non-Hodgkin lymphomas: results from the phase 1/2 BRUIN study. EHA 2021, EP500
- Normant E et al. TG-1710 a novel, orally available, and covalently-bound BTK inhibitor. EHA 2018, PF638
- Ribeiro M et al., TG-1701, a novel irreversible Bruton’s kinase inhibitor, cooperates with ublituximab-driven ADCC and ADCP in in vitro and in vivo models of ibrutinib-resistant mantle cell lymphoma. AACR 2020, 2205
- Cheah CY et al., Updated results of the selective Bruton tyrosine kinase inhibitor TG-1701, as monotherapy and in combination with ublituximab and umbralisib in patients with B-cell malignancies. J Clin Oncol 39, 2021 (suppl 15; abstr 7525)
- Herbaux C et al., Atezolizumab + obinutuzumab + venetoclax in patients with relapsed or refractory indolent non-Hodgkin’s lymphoma: primary analysis of a phase 2 trial from LYSA. EHA 2021, S212
- Cheah CY et al., Preliminary safety data from patients with relapsed/refractory B-cell malignancies treated with the novel B-cell lymphoma inhibitor BGB-11417. EHA 2021, EP525
- Hu N et al., Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with superior antitumor activities in haematological tumor models. AACR 2020, abstract 3077
- Data on file
- Scholz C et al., Phase 3 study of tafasitamab + lenalidomide and rituximab vs placebo + lenalidomide and rituximab in patients with relapsed/refractory follicular lymphoma or marginal zone lymphoma: inMIND. EHA 2021, PB1572
© 2021 Springer-Verlag GmbH, Impressum