Early-phase trials investigating novel agents in miscellaneous B-cell malignancies
Large B-cell lymphoma: subcutaneous epcoritamab
The prognosis is still poor for most patients with relapsed or refractory large B-cell lymphoma (LBCL) irrespective of therapeutic advances that have recently been achieved. There is a need for convenient, efficacious, well-tolerated, and readily available treatment options. Epcoritamab, a subcutaneously administered bispecific antibody, has been designed to simultaneously bind to CD3 and CD20, thus inducing T-cell–mediated cytotoxic activity against CD20-positive, malignant B cells. The phase I/II EPCORE NHL-1 study was initiated to establish the recommended phase II dose and to assess the safety and efficacy of subcutaneous epcoritamab, as well as pharmacokinetics and immune biomarkers, in patients with relapsed or refractory CD20-positive B-cell non-Hodgkin lymphoma (NHL). In the dose-escalation part, notable single-agent activity with clinically meaningful overall response rates (ORRs) and complete responses (CRs) was found, as well as a manageable safety profile [1].
Thieblemont et al. reported pivotal dose-expansion findings in patients with relapsed/refractory LBCL at EHA 2022 [2]. This cohort included 157 individuals with diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B. They had received ≥ 2 prior lines of antineoplastic therapy, including ≥ 1 anti-CD20 antibody.
This was a challenging-to-treat and highly refractory population. Primary refractory disease was present in 61 %, and 71 % had already been treated with ≥ 3 lines of therapy. Prior CAR-T cell therapy had been performed in 39 %, with 75 % of patients progressing within 6 months thereafter. The study treatment consisted of epcoritamab 48 mg QW in cycles 1-3, Q2W in cycles 4-9, and Q4W from cycle 10. ORR by independent review committee constituted the primary endpoint.
Deep and durable complete remissions
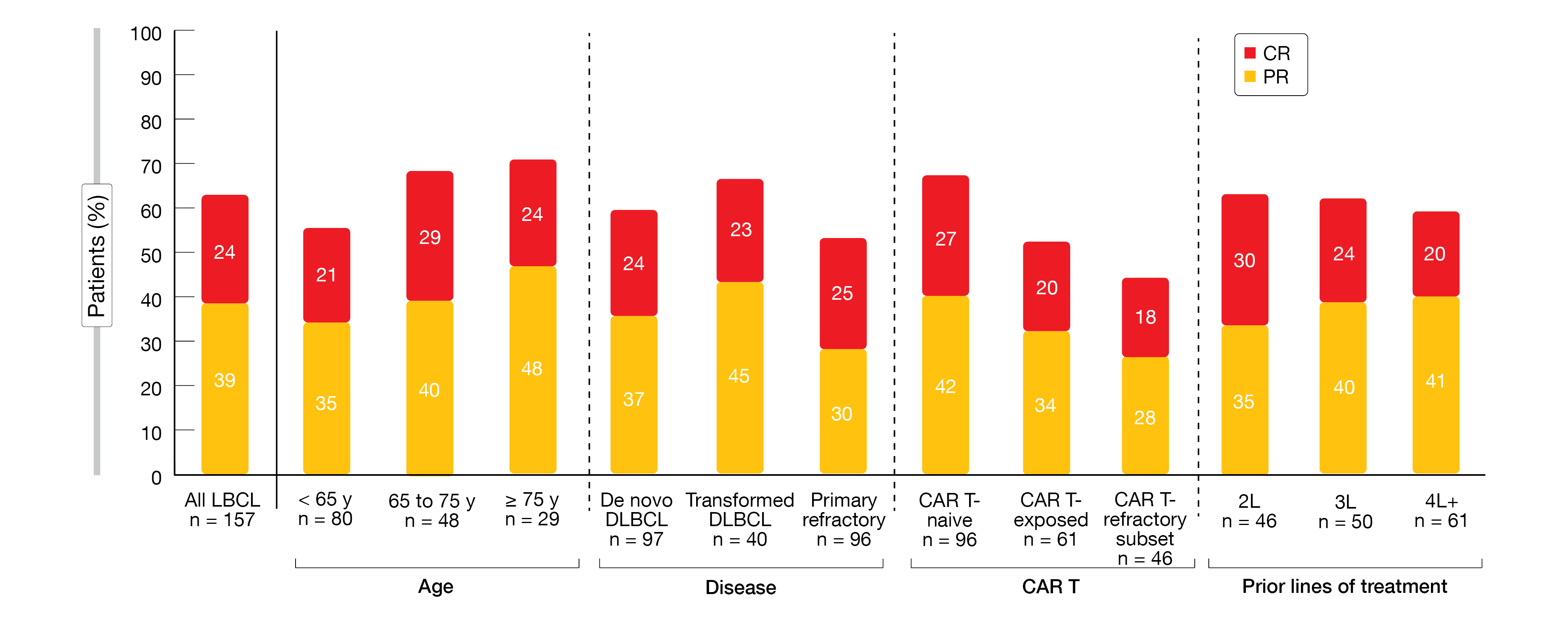
Single-agent epcoritamab demonstrated high response rates, with an ORR of 63 % and CRs in 39 %. Deep responses were achieved independent of age, disease subgroups, previous CAR-T therapy and response to it, and treatment lines (Figure 1). Epcoritamab was shown to drive deep and durable CRs. The majority of CRs were obtained by the time of the first or second assessment; however, some conversions from partial to complete remissions still took place at ≥ 36 weeks. The median duration of response for patients in CR had not been reached yet. At 9 months, 89 % of complete responders remained in CR.
Progression-free survival (PFS) varied according to best response; in the group with CR, it had not been reached yet, while it was 4.4 months in the entire group. Overall survival (OS) data were immature, with a 12-month OS rate of 56.9 %. Minimal residual disease (MRD) negativity resulted in 45.8 % in the total population. An exploratory ctDNA analysis demonstrated that MRD-negative responses were durable and correlated with PFS.
Moreover, epcoritamab was shown to be well tolerated. Most adverse events (AEs) were low grade and occurred early in treatment, in cycles 1-3. Among treatment-emergent AEs, cytokine-release syndrome was observed most commonly, but was mainly grade 1/2 and predictable, with events mainly seen after the first full dose. Ten patients (6.4 %) experienced immune effector cell-associated neurotoxicity syndrome. In 9 cases, these were grade 1 and 2 and resolved, while 1 patient died, which was confounded by multiple factors. The analysis yielded few discontinuations due to AEs (7 %); 32 % of patients remained on treatment. In all, epcoritamab is well tolerated and drives deep and durable responses in challenging-to-treat, highly refractory patients with relapsed/refractory LBCL.
Figure 1: Deep responses with subcutaneous epcoritamab across key subgroups in the EPCORE NHL-1 study
Phase II data on nemtabrutinib
BTK inhibitors are highly effective against several B-cell malignancies, although their use is limited by AEs, potentially due to off-target inhibition of other kinases. Also, BTK mutations can abrogate the binding capacity of the drug, resulting in resistance that might limit subsequent treatment options [3-5]. Nemtabrutinib is a potent, reversible inhibitor of both wild-type and ibrutinib-resistant C481S-mutated BTK [6]. The phase I/II dose-escalation and dose-expansion BELLWAVE-001 study is investigating nemtabrutinib in pretreated patients with CLL/SLL and various types of B-cell NHL. Preliminary results from the dose-expansion phase after a median follow-up of 6.18 months revealed promising antitumor activity and manageable safety of nemtabrutinib 65 mg QD in patients with pretreated CLL/SLL [7].
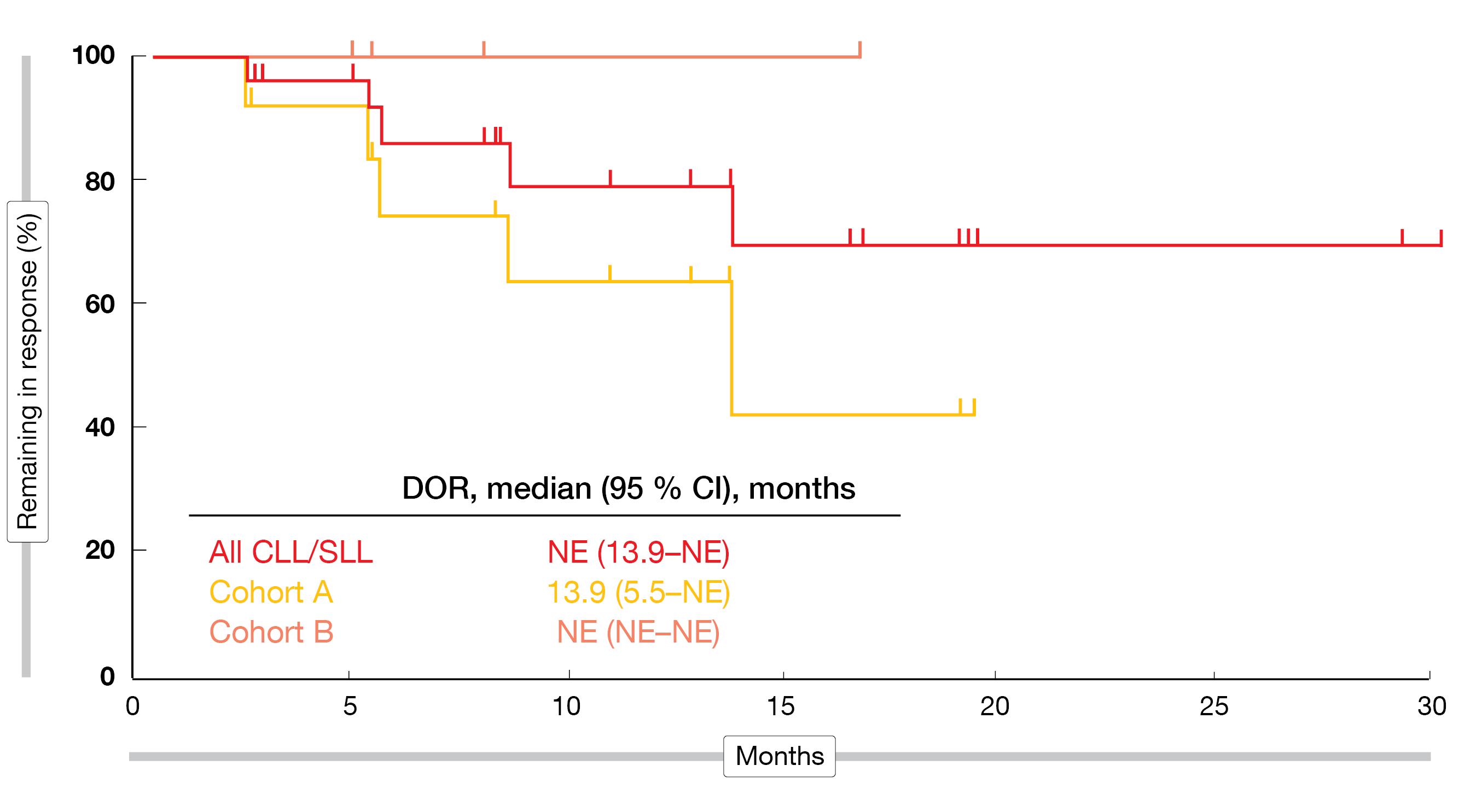
According to the update reported at EHA 2022, nemtabrutinib continued to show favorable activity, with responses observed in heavily pretreated patients and those progressing on prior covalent BTK inhibitors [8]. The ORR was 53 % in the entire cohort of 57 CLL/SLL patients treated with 65 mg QD. In Cohort A, which comprised patients with C481S mutation who had received ≥ 2 prior therapies including covalent BTK inhibitors (n = 25), 60 % of patients responded; in Cohort B including patients without C481S mutation after ≥ 2 prior therapies and intolerance to BTK inhibitors (n = 10), the ORR was 40 %. Median duration of response had not been reached in all patients and in Cohort B, while it was 13.9 months in Cohort A (Figure 2). Likewise, median PFS was 15.7 months in Cohort A and had not been reached yet in the other groups.
AEs proved manageable, with the most common grade ≥ 3 treatment-emergent AEs including decreased neutrophil counts (27 %), thrombocytopenia, hypertension, and pneumonia (14 % each). Two patients developed atrial fibrillation that was grade ≥ 3 in 1 case. The investigation of nemtabrutinib at doses ≥ 65 mg for the treatment of B-cell malignancies is continuing.
Figure 2: BELLWAVE-001 study: duration of response on nemtabrutinib treatment in all patients and in Cohorts A and B
Zanubrutinib after acalabrutinib intolerance
The ongoing, multicenter, single-arm, phase II BGB-3111-215 study is assessing the safety and efficacy of the next-generation BTK inhibitor zanubrutinib in patients with CLL/SLL, Waldenström’s macroglobulinemia (WM), mantle cell lymphoma (MCL), or marginal zone lymphoma who have discontinued ibrutinib and/or acalabrutinib treatment due to AEs. Previous findings demonstrated that zanubrutinib was well tolerated in this patient group [9]. At EHA 2022, Shadman et al. presented updated results for cohort 2 of the study that included acalabrutinib-intolerant patients [10]. The safety of zanubrutinib was assessed based on the recurrence and change in severity of AEs that had led to acalabrutinib intolerance.
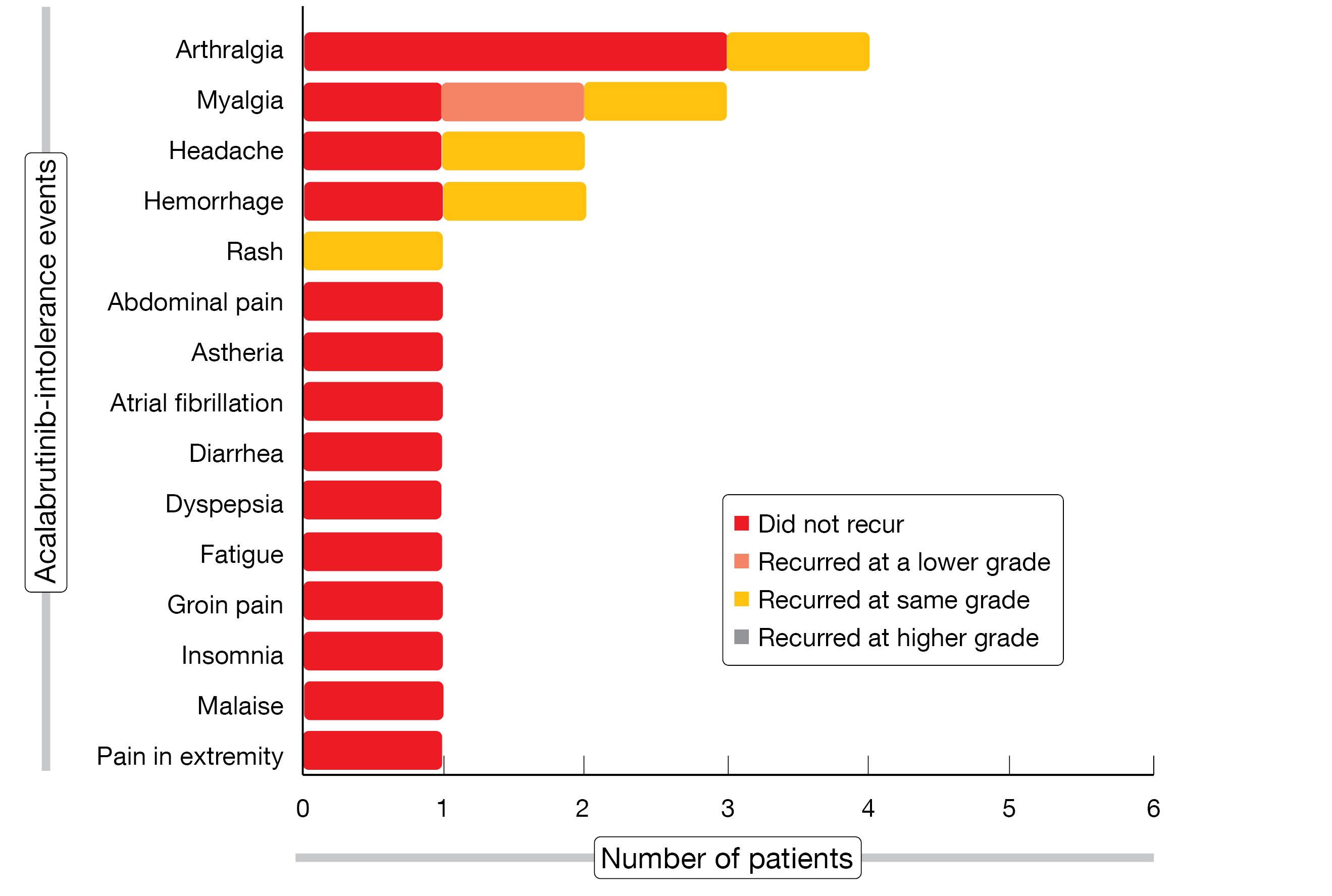
The results obtained in 13 patients showed that 73 % of acalabrutinib-related intolerance events did not recur on zanubrutinib treatment (Figure 3), which meant that 62 % of patients did not experience recurrence of any event. Among 6 events that recurred, 5 were rated as the same grade and 1 as lower grade. One patient discontinued treatment due to recurrence of myalgia of the same grade. Three patients who experienced the same intolerance events on ibrutinib and acalabrutinib (i.e., pain in extremity, diarrhea, and atrial fibrillation) did not develop recurrence of these on zanubrutinib therapy. Eighty percent of the 10 zanubrutinib-treated patients with ≥ 90 days of follow-up achieved at least stable disease, and 70 % experienced deepening of response. The authors concluded that zanubrutinib might be a viable therapeutic option for patients who are intolerant to acalabrutinib. Enrollment and follow-up of the study are ongoing.
A novel strategy to overcome on-target resistance mutations emerging from BTK inhibitor treatment is the use of BGB-16673, an investigational BTK-targeting chimeric degradation activation compound active against both wild-type and mutant BTK [11]. The phase I, open-label, dose-escalation and dose-expansion study BGB-16673-101 will assess BGB-16673 as the first-in-human study (NCT05006716). Adult patients with select relapsed/refractory B-cell malignancies are eligible. After determination of the recommended phase II dose, Cohort 1 will contain patients with relapsed/refractory CLL/SLL after BTK inhibition, while Cohort 2 will enroll those with relapsed/refractory MCL post BTK inhibitor treatment.
Figure 3: Recurrence and severity of acalabrutinib-related intolerance events on treatment with zanubrutinib
BGB-11417 as monotherapy and plus zanubrutinib in phase I
Treatment with the BCL2 inhibitor venetoclax can be impeded by gastrointestinal toxicities, neutropenia, and the emergence of specific BCL2 mutations resulting in resistance [12, 13]. The potent and highly selective BCL2 inhibitor BGB-11417 has shown more pronounced antitumor activity than venetoclax in human acute lymphatic leukemia, MCL and DLBCL in xenograft mouse models [14]. In addition, BGB-11417 has a broad therapeutic index and tolerable safety profile.
The first-in-human, open-label, multicenter, dose-escalation and dose-expansion phase I trial BGB-11417-101 investigated BGB-11417 as monotherapy (parts 1 and 2) and in combination with zanubrutinib (parts 3 and 4) in patients with NHL, WM, and CLL/SLL. All patients had relapsed/refractory disease except for those with CLL/SLL who were divided into a treatment-naïve and a relapsed/refractory cohort. Disease-specific dose-escalation cohorts assessing up to 5 potential dose levels of BGB-11417 (i.e., 40 mg, 80 mg, 160 mg, 320 mg, 640 mg QD) are followed by the corresponding expansion cohorts.
At the time of the cutoff for the preliminary analysis reported at EHA 2022, 78 patients had been treated, with 34 and 44 in the monotherapy and combination cohorts, respectively [15]. Transient neutropenia was the most common grade ≥ 3 AE; overall, grade ≥ 3 AEs were infrequent and manageable. The risk of tumor lysis syndrome appeared limited, with laboratory TLS observed in only 1 CLL patient with high TLS risk who received monotherapy. The available data indicated that the combination of BGB-11417 with zanubrutinib is well tolerated, similar to single-agent BGB-11417, at the dose levels tested.
With respect to efficacy, responses were observed at the preliminary dose levels as dose escalation had not yet been completed for any cohort. Significant reductions in absolute lymphocyte counts occurred during ramp-up for all patients with CLL. In the relapsed/refractory setting, the combination treatment gave rise to promising early response rates, with 80 % of patients achieving partial response with rebound lymphocytosis or better across the dose levels ranging from 40 to 320 mg.
REFERENCES
- Hutchings M et al., Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 2021; 398(10306): 1157-1169
- Thieblemont C et al., Subcutaneous epcoritamab in patients with relapsed or refractory large B-cell lymphoma (EPCORE NHL-1): pivotal results from a phase 2 study. EHA 2022, LB2364
- Preetesh J et al., Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol 2018; 183(4): 578-587
- Xu L et al., Acquired mutations associated with ibrutinib resistance in Waldenström macroglobulinemia. Blood 2017; 129(18): 2519-2525
- Woyach J et al., Resistance to acalabrutinib in CLL is mediated primarily by BTK mutations. Blood 2019; 134(suppl_1): 504
- Woyach J et al., Final results of phase 1, dose escalation study evaluating ARQ 531 in patients with relapsed or refractory B-cell lymphoid malignancies. Blood 2019; 134: 4928
- Woyach JA et al., Preliminary efficacy and safety of MK-1026, a non-covalent inhibitor of wild-type and C481S mutated Bruton tyrosine kinase, in B-cell malignancies: a phase 2 dose expansion study. Blood 2021; 138: 392
- Woyach J et al., Nemtabrutinib (MK-1026), a noncovalent inhibitor of wild-type and C481S-mutated Bruton tyrosine kinase for B-cell malignancies: efficacy and safety of the phase 2 dose-expansion BELLWAVE-001 study. EHA 2022, P682
- Shadman M et al., Phase 2 study of zanubrutinib in BTK inhibitor-intolerant patients with relapsed/refractory B-cell malignancies. Blood 2021; 138(suppl 1): 1410
- Shadman M et al., Zanubrutinib in acalabrutinib-intolerant patients with B-cell malignancies. EHA 2022, PB1890
- Tam CS et al., A phase 1 first-in-human study of BGB-16673, a Bruton tyrosine kinase protein degrader, in patients with B-cell malignancies (Trial in Progress). EHA 2022, P686
- Davids et al., Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res 2018; 24(18): 4371-4379
- Blombery P et al., Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov 2019; 9(3): 342-353
- Hu N et al., Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with superior antitumor activities in haematological tumor models. AACR 2020, abstract 3077
- Opat S et al., A phase 1 study with the novel BCL2 inhibitor BGB-11417 as monotherapy or in combination with zanubrutinib in patients with B-cell malignancies: preliminary data. EHA 2022, P687
© 2022 Springer-Verlag GmbH, Impressum