Updates and ancillary analyses in the setting of chronic lymphocytic leukemia
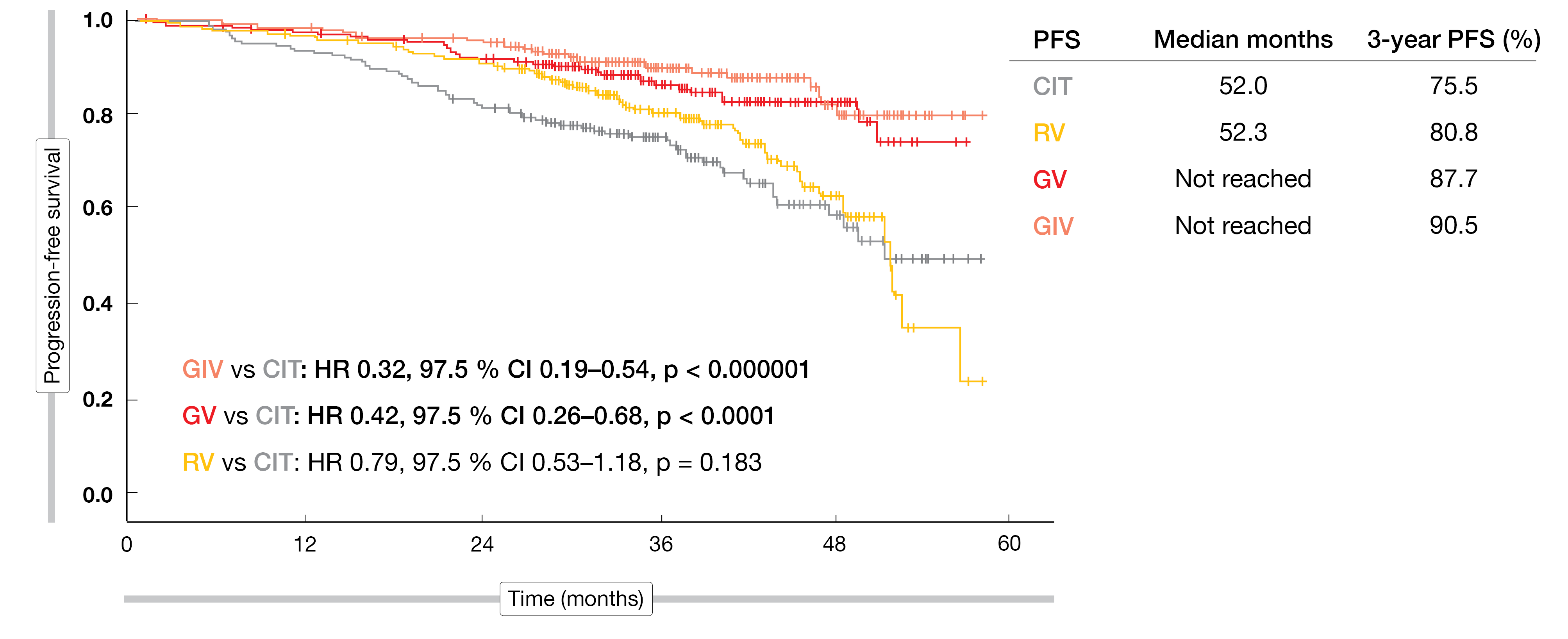
Progression-free survival in GAIA/CLL13
In fit patients with advanced CLL of favorable genetic risk, chemoimmunotherapy (CIT) consisting of fludarabine/cyclophosphamide/rituximab (FCR) or bendamustine/rituximab (BR) still represents the treatment standard. Another potential strategy, however, is time-limited therapy consisting of obinutuzumab plus venetoclax (GV) with or without a BTK inhibitor. The four-arm, randomized, phase III GAIA/CLL13 trial compared GV and GV plus ibrutinib (GIV) to 6 cycles of FCR (patient age ≤ 65 years) or BR (> 65 years) in fit patients (i.e., CIRS scores ≤ 6 and normal creatinine clearance) in whom TP53 mutation and deletion 17p had been excluded. In the fourth study arm, rituximab plus venetoclax (RV) was administered. Overall, the trial contained 926 patients. The regimens were administered for a maximum of 12 cycles; only ibrutinib was allowed to be continued for up to 36 cycles in case of minimal residual disease (MRD) persistence.
The coprimary endpoint of undetectable MRD (uMRD) at 15 months in peripheral blood has been presented at the ASH 2021 congress [1]. This showed significantly higher uMRD rates with GIV and GV compared to CIT (p < 0.0001 each), while RV was not superior to CIT. At EHA 2022, Eichhorst et al. reported the second coprimary endpoint of the GAIA/CLL13 trial, which was interim progression-free survival (PFS) for GIV vs. CIT, as well as other study outcomes [2].
Broad clinical benefits with GV-based treatment
In keeping with the uMRD results, the PFS interim analysis demonstrated superior results for both GV-based regimens vs. CIT after a median follow-up of 38.8 months (Figure 1). Median PFS had not been reached for GIV or GV, while it was 52 months for both CIT and RV. At 3 years, 90.5 % and 87.7 % of the patients treated with GIV and GV, respectively, were progression-free (vs. 75.5 % with CIT). GV-based treatment prolonged PFS in almost all subgroups compared to CIT. The analysis according to the IGHV mutation status showed that PFS benefits due to GIV and GV occurred particularly in the unmutated IGHV setting, with rates of 86.6 % and 82.9 % vs. 65.5 % for CIT. In the group with mutated IGHV, the treatments showed no considerable differences. Patients aged ≤ 65 years in the mutated IGHV cohort derived equal PFS benefits from CIT, GIV, and GV therapy.
Time to next treatment (TTNT) was longer with the GV-based regimens than with CIT. At 3 years, 98.3 % and 94.1 % of patients in the GIV and GV arms, respectively, had not received any subsequent therapy (vs. 87.2 % with CIT). No differences in overall survival (OS) were observed between the study arms, although the overall number of events and the follow-up were still immature.
Grade ≥ 3 AEs occurred with similar incidence in the GIV, GV and CIT arms (83.5 %, 84.2 % and 81.5 %, respectively). The lower rate observed with RV (73.0 %) was particularly due to fewer blood and lymphatic system AEs. Rates for infections were highest with CIT (20.4 %) and GIV (22.1 %), which also applied to febrile neutropenia (11.1 % and 7.8 %, respectively). Hypertension, which is known to be an ibrutinib-specific AE, was noted in 5.6 % in the GIV arm. Second primary malignancies showed an increased incidence after CIT (49 cases vs. 29, 27 and 24 with GIV, GV, and RV, respectively). This was mainly based on a larger number of solid tumors and non-melanoma skin cancers. For Richter transformation, no distinct differences were noted across the four arms.
Figure 1: GAIA/CLL13: superior progression-free survival with GV-based regimens vs. RV and CIT
CLL14: 5-year update
In contrast to CLL13, the CLL14 study was designed to assess first-line treatment with GV in patients who had coexisting medical conditions as evidenced by CIRS scores > 6 and/or creatinine clearance < 70 mL/min. In the experimental arm, patients were treated with GV for 6 cycles followed by venetoclax monotherapy for another 6 cycles. Those in the control arm received 6 cycles of chlorambucil plus obinutuzumab followed by 6 cycles of chlorambucil. Each arm contained 216 patients. PFS was defined as the primary endpoint. At EHA 2022, Al-Sawaf et al. reported the 5-year analysis of CLL14 after a median observation time of 65.4 months [3].
At least 4 years after treatment cessation, the median PFS had still not been reached with GV and was 36.4 months with chlorambucil/obinutuzumab. The 5-year PFS rates amounted to 62.6 % vs. 27.0 %, translating into a 65 % reduction in the risk of progression or death (HR, 0.35; p < 0.0001). In the experimental arm, median PFS had not been reached yet in patients without TP53 deletion and/or mutation, and was 49.0 months in those with these risk factors. Patients in the control arm showed median PFS of 38.9 and 19.8 months, respectively, for the two subgroups. Similarly, PFS was longest in the IGHV-mutated group treated with GV (not reached) followed by the GV-treated patients with unmutated IGHV (64.2 months), whereas the patients on chlorambucil/obinutuzumab experienced poorer PFS outcomes across the IGHV subgroups (59.9 and 26.9 months, respectively). These findings imply that the combined fixed-duration approach is feasible even in the presence of high-risk genomics.
A multivariable model identified pretreatment disease burden and deletion 17p as independent prognostic factors for PFS in the context of GV treatment. For pretreatment disease burden, the threshold was defined as a maximum lymph node size of > 5 cm and absolute lymphocyte counts > 25 G/L. Prognostic variables for chlorambucil/obinutuzumab, on the other hand, included IGHV mutational status, deletion 17p, deletion 11q, complex karyotype, and serum β2 microglobulin.
Deeper and sustained MRD rates after GV
Median TTNT had not been reached with GV and was 52.9 months with chlorambucil/obinutuzumab. At 5 years, 72.09 % vs. 42.84 % of patients were alive and had not received any further line of therapy (HR, 0.42; p < 0.0001). When viewed according to genomic risk groups, the course of disease was again more favorable in the absence of high-risk features, with TTNT being longest in the GV-treated cohort.
Second-line treatments in both arms comprised mainly targeted agents including several BTK inhibitors, although 26-31 % of patients received chemo(immuno)therapy, which reflects the limited access to second-line targeted drugs and can be expected to confound the OS observations to a certain degree. Median OS had not been reached yet; at 5 years, 81.9 % vs. 77.0 % of patients were alive (HR, 0.72). Only 8 patients in the experimental arm died due to CLL-related causes (vs. 23 in the control arm), which suggests that upfront use of BCL2 inhibitors can effectively contribute to controlling CLL-related mortality.
The updated analysis yielded no new safety signals after the prolonged follow-up. After the end of treatment, the AE rates remained very low. The cumulative incidence of second primary malignancies did not differ significantly between the two study arms.
Four years after treatment discontinuation, 18.1 % vs. 1.9 % of patients had sustained MRD < 10-4 in the peripheral blood. The depth of remission beyond 10-4 was shown to significantly correlate with long-term PFS, thus indicating the value of ultra-sensitive MRD assessments. Likewise, within the GV-treated group, patients with MRD ≥ 10-4 had a shorter OS than those with MRD < 10-4; this highlights the need for dedicated MRD-guided approaches. Tailored strategies need to be developed for patients who remain MRD-positive after GV treatment.
MRD endpoint of the FLAIR study
The phase III NCRI FLAIR trial was initiated to compare ibrutinib plus rituximab with FCR in untreated, fit CLL patients. Indeed, the combination gave rise to improved PFS [4]. Two additional arms were added later on that tested ibrutinib plus venetoclax against ibrutinib monotherapy, the primary endpoint being MRD eradication. This population was ≤ 75 years old, considered fit for FCR, and was previously untreated but required therapy by IWCLL criteria. In patients who achieved MRD negativity, ibrutinib was not stopped immediately but was continued for the same duration that had passed until the negative assessment, to ensure further cell reduction and to enhance the likelihood of cure. Patients with MRD negativity in the peripheral blood underwent repeat testing after 3 months as well as blood and bone marrow testing at 6 months; if all of these assessments were negative, the first result was considered the time to MRD negativity.
The patients in the ibrutinib/venetoclax arm (n = 136) were treated for 2-6 years depending on the time to MRD negativity. Ibrutinib monotherapy (n = 138) was administered for a maximum of 6 years. If MRD positivity returned prior to year 6 according to periodic MRD measurements, ibrutinib was restarted. Hillmen et al. presented the interim data at EHA 2022 [5].
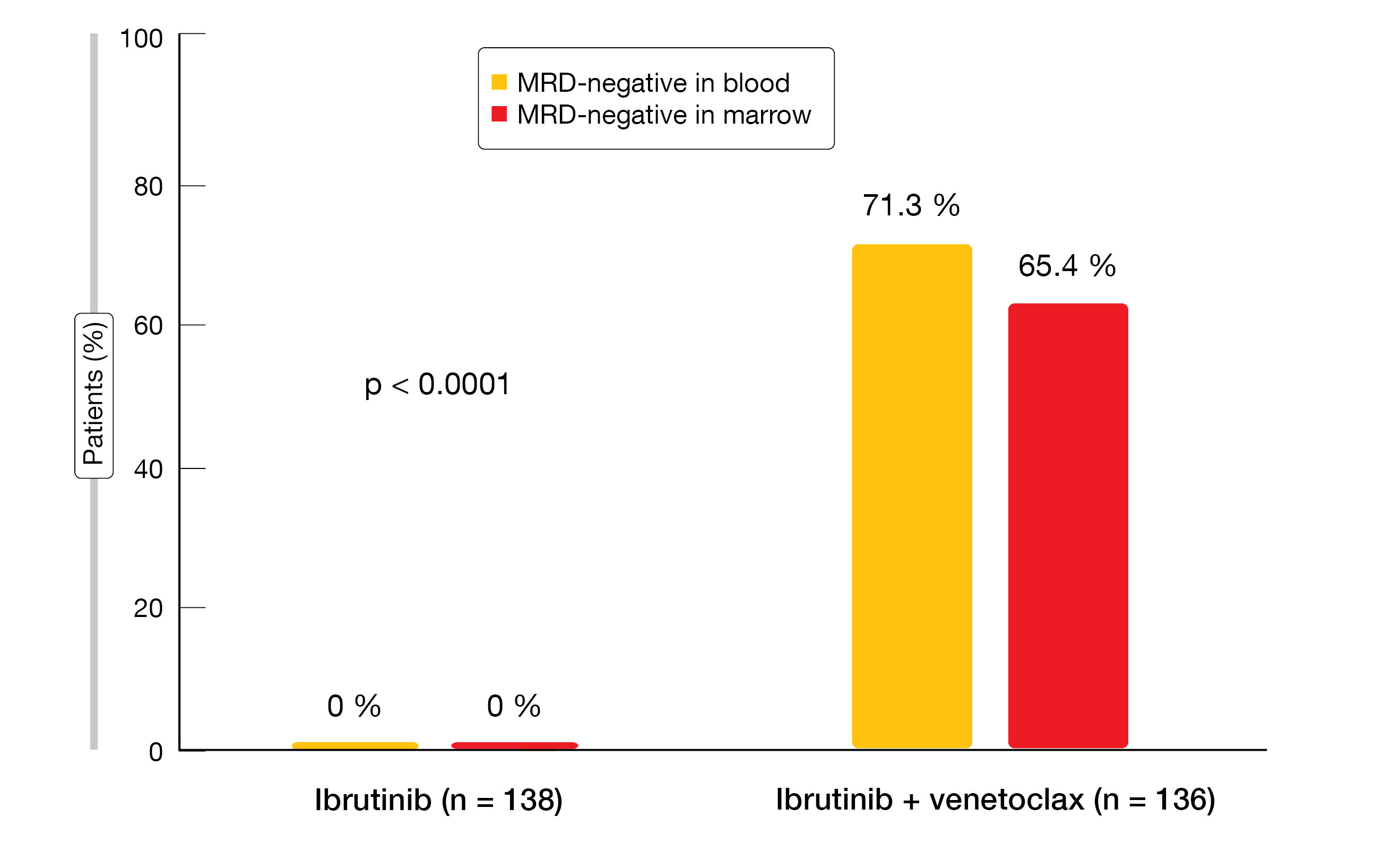
454-fold higher chance of achieving MRD negativity
Regarding the primary endpoint, which was MRD negativity at 2 years, the NCRI FLAIR trial revealed significant superiority of ibrutinib/venetoclax. MRD negativity was achieved in the blood and bone marrow in 71.3 % and 65.4 %, respectively, whereas none of the ibrutinib-treated patients had turned MRD-negative after 2 years’ time (Figure 2). Due to this highly significant difference (p < 0.0001), penalized logistic regression models were fitted to enable finite parameter estimation. This showed that the odds of achieving MRD negativity in the bone marrow were 454 times higher for patients receiving the combination than for those treated with ibrutinib monotherapy. Median time to MRD eradication was 12 and 19 months in the blood and bone marrow, respectively, for ibrutinib/venetoclax, and had not been reached with ibrutinib in either compartment. Almost 43 % patients treated with the combination stopped therapy due to meeting MRD stopping rules (vs. 0 % in the control arm).
While factors such as age and gender did not affect the outcomes, mutational status did. In the combination arm, MRD negativity was obtained more frequently by IGHV-unmutated than IGHV-mutated patients (79.7 % vs. 56.4 %). MRD eradication emerged in 82.8 % of patients with 11q deletion but only in 54.5 % of those with 13q deletion. The IWCLL response 9 months after randomization constituted a secondary endpoint. Approximately 90 % of the entire study population responded, although the patients in the experimental arm achieved a considerably higher CR rate than those in the control arm (59.6 % vs. 8 %).
The combination was well tolerated, with higher rates of diarrhea, nausea, anemia and leukopenia in ibrutinib/venetoclax-treated patients. Among grade ≥ 3 AEs, only leukopenia was notably increased in the experimental arm (27.4 % vs. 5.1 %). No cases of clinical tumor lysis syndrome occurred; laboratory TLS was reported in 4.4 % in the combination arm vs. 0 % in the control arm.
Figure 2: MRD negativity with ibrutinib/venetoclax vs. ibrutinib at 2 years in the NCRI FLAIR trial
BRUIN: pirtobrutinib after BTK failure
Although BTK inhibitors have revolutionized the outcomes in leukemia, resistance and intolerance limit their clinical benefits. Ibrutinib discontinuation rates at 5 years have been estimated at 41 % and 54 % for the frontline and relapsed/refractory settings, respectively [6, 7]. Available options following covalent BTK inhibitor treatment are limited.
The phase I/II BRUIN study is evaluating the highly selective, potent, non-covalent BTK inhibitor pirtobrutinib (LOXO-305) in patients with previously treated, advanced B-cell malignancies. The analysis presented by Mato et al. at EHA 2022 related to the group of 261 BTK-inhibitor–pretreated individuals with CLL/small lymphocytic lymphoma (SLL) [8]. In addition to BTK inhibition, most of them had previously received anti-CD20 antibody therapy and chemotherapy. BCL2 inhibitors had been administered in 41 %. Furthermore, high-risk molecular characteristics were present in a considerable proportion of patients.
Pirtobrutinib demonstrated promising efficacy in this heavily pretreated population, with an overall response rate (ORR) of 68 %. Responses were shown to be independent of the BTK C481 status, the reason for prior BTK discontinuation (i.e., progression vs. intolerance), and other classes of prior therapy including covalent BTK inhibitors, BCL2 inhibitors, and PI3Kδ inhibitors. Also, the responses appeared to deepen over time. The ORR increased to 73 % in the patient group with ≥ 12 months of follow-up.
In terms of PFS, the treatment induced durable disease control. Median PFS in at least BTK-inhibitor–treated patients (prior lines, 3) had not been reached yet, and in those who had received at least prior BTK plus BCL2 inhibitor treatment (prior lines, 5), median PFS was 18 months. Seventy-four percent remained on pirtobrutinib therapy at the time of the analysis. The treatment with the novel BTK inhibitor was extremely well tolerated. Any-grade treatment-emergent AEs included fatigue (23 %), diarrhea (19 %), neutropenia (18 %), and contusion (17 %). AEs of special interest were rare and mainly low-grade. No dose-limiting toxicities occurred, and the maximum tolerated dose was not reached. Only 1 % of patients permanently discontinued therapy due to treatment-related AEs.
Pirtobrutinib plus venetoclax ± rituximab
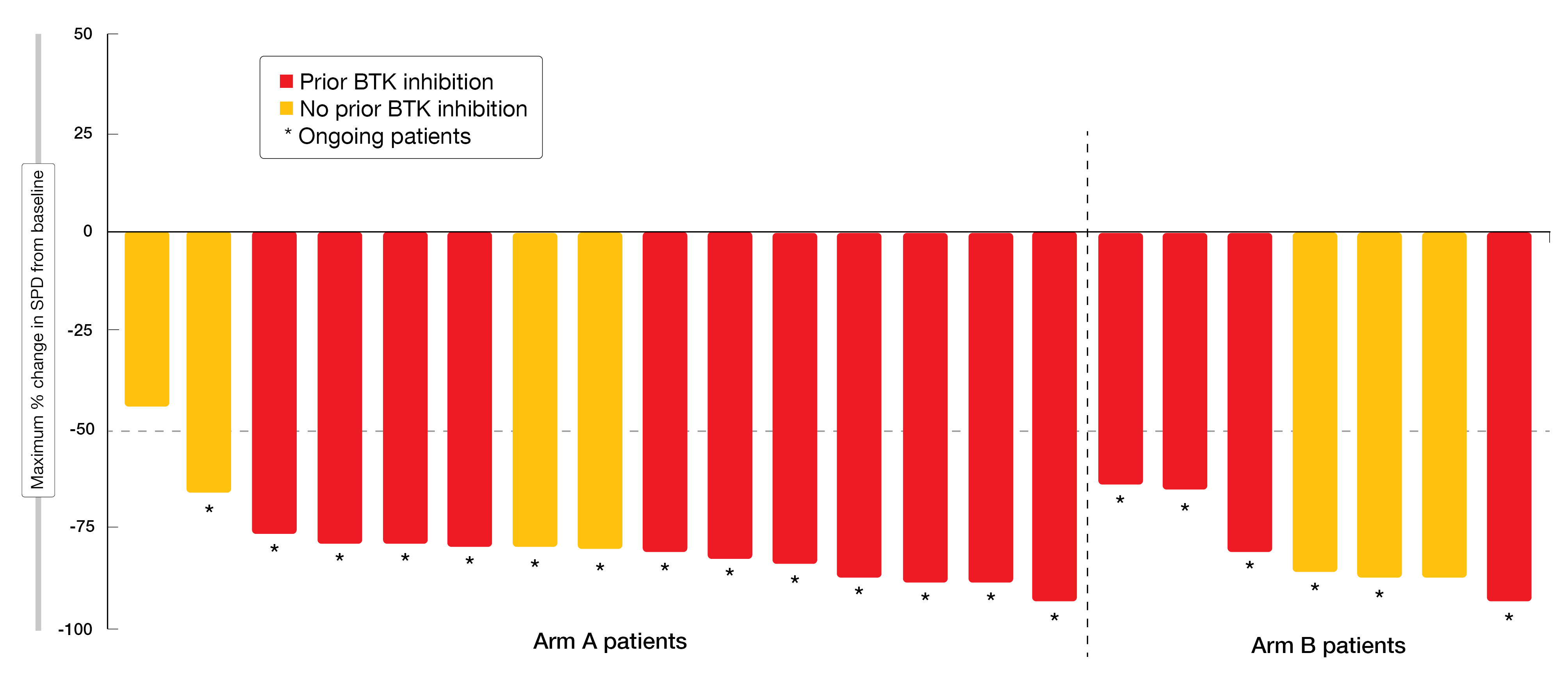
The phase IB part of the BRUIN study assessed pirtobrutinib-based combinations in patients with relapsed/refractory CLL. Fifteen individuals received pirtobrutinib plus venetoclax (Arm A), while 10 were treated with pirtobrutinib plus venetoclax and rituximab (Arm B). Early results reported at EHA 2022 indicated promising efficacy of the combination approach [9]. All patients experienced reductions in tumor size irrespective of BTK inhibitor pretreatment (Figure 3). Partial remissions were achieved in 93.3 % and 100 % in Arms A and B, respectively. The median time to best response was short at 1.9 across the two arms. All responding patients except for two remained on therapy at the time of the analysis. Median time on treatment was 9.8 and 6.0 months, respectively.
Pirtobrutinib plus venetoclax ± rituximab was well tolerated, with a safety profile consistent with known drug class findings and no clear additive toxicities in this patient group. The most common any-grade treatment-emergent AEs included fatigue, nausea, and decreased neutrophil counts in Arm A, as well as constipation, diarrhea, infusion-related reactions and decreased neutrophil counts in Arm B. No dose-limiting toxicities occurred, and no patient discontinued treatment due to drug-related AEs. According to the pharmacokinetics assessed, there was no apparent interaction between pirtobrutinib ± rituximab and venetoclax. Randomized, global, phase III trials are currently evaluating pirtobrutinib in CLL/SLL as monotherapy and in combination with other targeted drugs.
Figure 3: Reductions in tumor size achieved with pirtobrutinib/venetoclax (Arm A) and pirtobrutinib/venetoclax plus rituximab (Arm B)
3-year data from CAPTIVATE
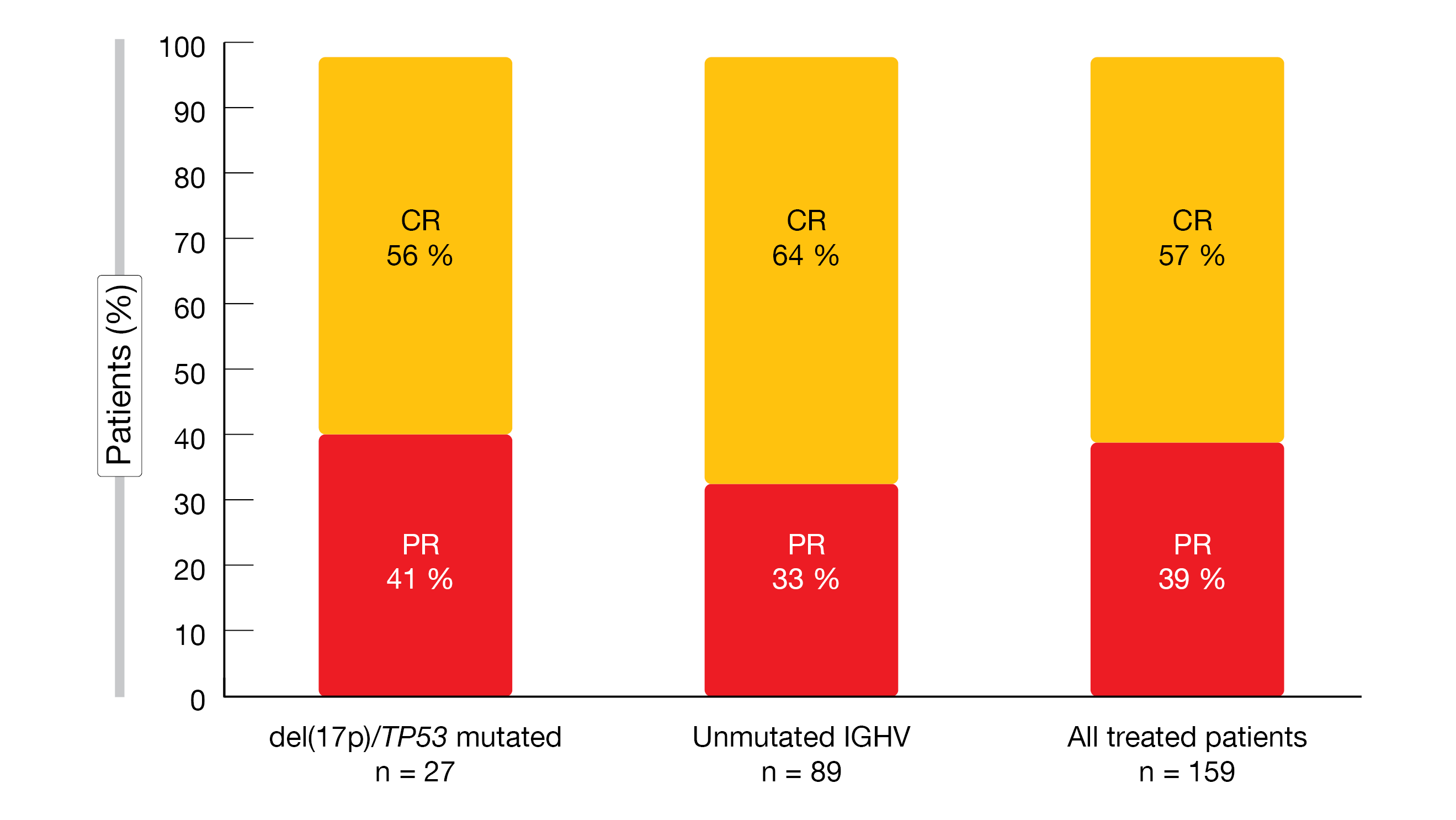
First-line treatment with 3 cycles of ibrutinib followed by 12 cycles of combined ibrutinib and venetoclax was evaluated by the international, phase II CAPTIVATE trial. The open-label, single-arm FD Cohort of the study assessed the fixed-duration administration of the combination for 12 cycles after a 3-cycle ibrutinib lead-in. The primary endpoint, which was the CR rate including complete response with incomplete bone marrow recovery (CRi) per investigator assessment in patients with del(17p), was met [10]. CR/CRi resulted in 56 %, with similarly high rates overall and in patients with high-risk features. Wierda et al. presented the 3-year follow-up of the FD Cohort after a median time on the study of 38.7 months, which included a median of 24.9 months after completion of treatment [11].
The data revealed that the CR rate had increased from 55 % at the time of the primary analysis to 57 %. Similar results were obtained for patients with deletion 17/TP53 mutation and unmutated IGHV (Figure 4). Median duration of CR had not been reached, with the 24-month estimate being 94 %. Seventy-nine percent of patients had a best response of uMRD in blood and/or bone marrow. Within the group of those with uMRD in the blood 3 months after treatment, 78 % of evaluable patients maintained uMRD through another 9 months.
The 36-month PFS rate was as high as 88 % overall, with similar rates in patients with del(17p)/TP53 mutation (80 %) and unmutated IGHV (86 %). No patient died during the prolonged follow-up. At 36 months, 98 % of the total population were alive. Again, the 36-month rates for OS were similar for patients with del(17p)/TP53 mutation and unmutated IGHV (96 % and 97 %, respectively). Twelve patients who progressed after the combination received retreatment with single-agent ibrutinib. Nine of 11 evaluable patients achieved partial response, and one each obtained partial response with lymphocytosis and disease stabilization. Successful re-treatment thus appears to be feasible. The safety profile of the ibrutinib/venetoclax combination proved manageable and unchanged from that previously reported, with the majority of AEs being mild and resolving quickly.
The authors noted in their summary that the treatment continued to demonstrate deep and durable responses, as well as clinically meaningful PFS, including in patients with high-risk features. Ibrutinib/venetoclax represents an all-oral, once-daily, chemotherapy-free, fixed-duration regimen for previously untreated patients with CLL/SLL.
Figure 4: Best overall response to fixed-duration treatment with ibrutinib/venetoclax in the CAPTIVATE study 2 years after the end of treatment
ELEVATE TN: significant OS benefit after 5 years
The randomized, open-label, phase III ELEVATE TN trial evaluated the next-generation BTK inhibitor acalabrutinib with or without obinutuzumab versus chlorambucil/obinutuzumab in treatment-naïve CLL patients who were aged ≥ 65 years or < 65 years and had CIRS scores > 6 or creatinine clearance of 30-69 mL/min. Both the acalabrutinib combination and the monotherapy improved PFS compared to the chemoimmunotherapy regimen at a median follow-up of 28.3 months (p < 0.0001 each) [12].
According to the 5-year follow-up presented at EHA 2022, efficacy and safety of acalabrutinib/obinutuzumab and single-agent acalabrutinib were maintained [13]. Median PFS had not been reached yet with either regimen, resulting in 89 % and 79 % risk reductions with the combination and the monotherapy, respectively, compared to chlorambucil/obinutuzumab (HRs, 0.11 and 0.21, respectively; p < 0.0001 each). The estimated 60-month PFS rates were 84 %, 72 %, and 21 %, respectively. Particularly profound benefits were achieved in the subgroup with unmutated IGHV; here, the median PFS had not been reached for both acalabrutinib/obinutuzumab and single-agent acalabrutinib, while it was 22.2 months among patients in the chlorambucil/obinutuzumab arm, which gave rise to risk reductions of 94 % and 88 %, respectively (HRs, 0.06 and 0.12, respectively; p < 0.0001 each). At 60 months, 82 % and 72 % vs. 6 % of patients were progression-free.
Although median OS had not been reached yet in any of the treatment arms, the combination was shown to induce a significant benefit compared to chemoimmunotherapy (HR, 0.55; p = 0.0474), with 60-month OS rates of 90 %, 84 %, and 82 %. The treatment is ongoing in 65 % and 60 % of patients treated with acalabrutinib/obinutuzumab and single-agent acalabrutinib, respectively.
Long-term efficacy of acalabrutinib-based regimens
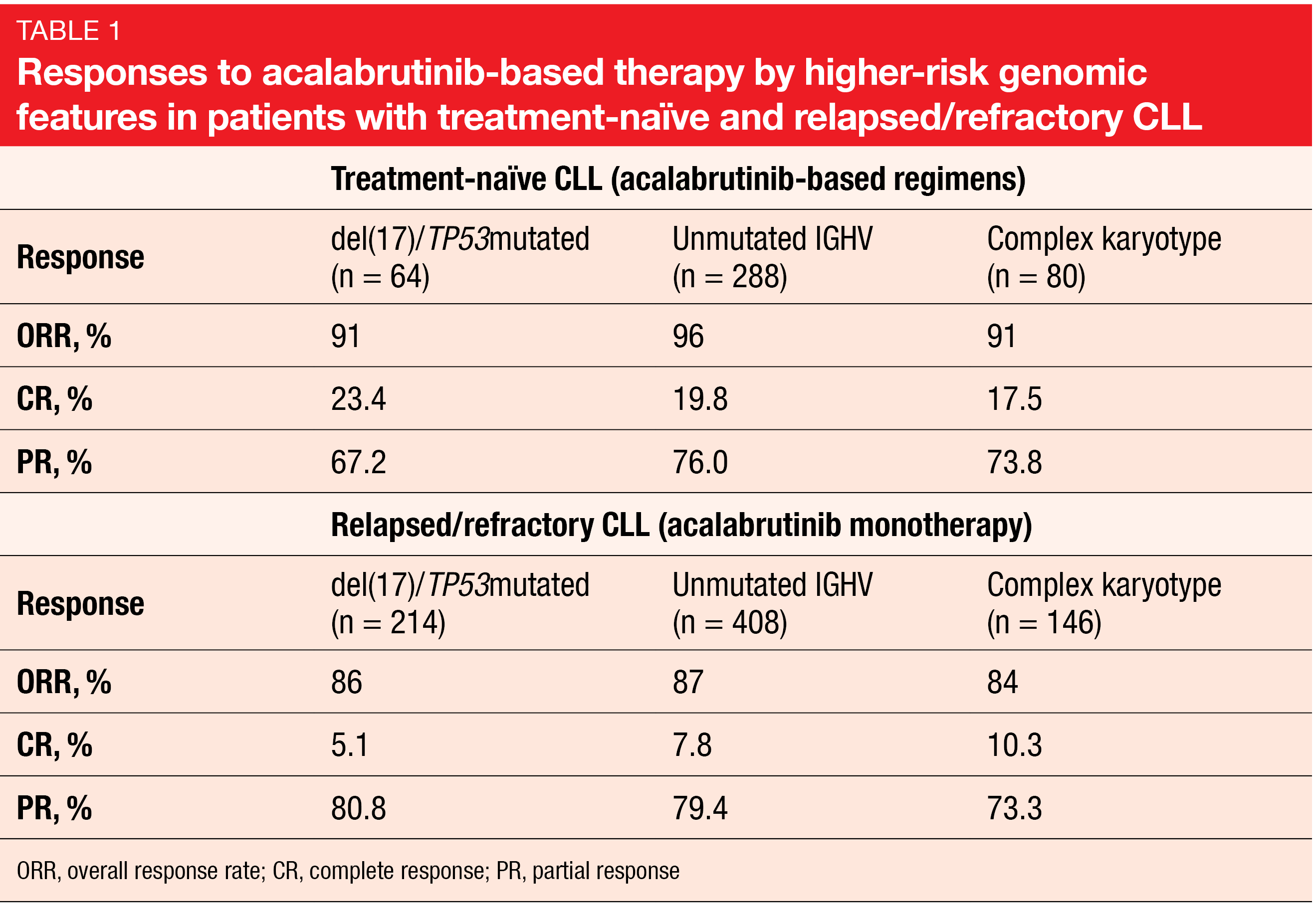
A pooled analysis of data from 7 clinical trials assessed the long-term efficacy of acalabrutinib-based regimens in patients with treatment-naïve or relapsed/refractory CLL and higher-risk genomic features including deletion 17p and/or TP53 mutation, unmutated IGHV, and complex karyotype [14]. The data of 809 patients treated with acalabrutinib or acalabrutinib/obinutuzumab were analyzed. Within this group, 321 and 488 had newly diagnosed and relapsed/refractory disease, respectively.
At a median follow-up of almost 4 years, PFS and OS rates were shown to be high in all genomic subgroups. In treatment-naïve patients, at 48 months, the PFS and OS rates ranged from 76 % to 91 % and 88 % to 95 %, respectively, depending on the genetic setup. At 36 months, 73 % to 82 % of patients with relapsed/refractory disease were alive, and 54 % to 65 % showed freedom from progression. The ORRs were consistent across the treatment-naïve and pretreated cohorts (Table 1). Despite the presence of higher-risk genomic features, discontinuation rates due to Richter’s transformation were low in both treatment-naïve and relapsed/refractory cohorts (0.3 % and 0.4 %, respectively).
The safety profile of acalabrutinib in this analysis was similar to the reported overall safety profile, with low rates of atrial fibrillation/flutter and major hemorrhage. At the time of the analysis, more than half of the patients in the treatment-naïve cohort remained on treatment, with up to 82 months of follow-up. Overall, these findings illustrate the long-term benefit of acalabrutinib-based regimens in patients with CLL and higher-risk genomic features regardless of treatment line.
HRQOL with first-line zanubrutinib: SEQUOIA
The international, open-label, randomized, phase III SEQUOIA trial examined the next-generation BTK inhibitor zanubrutinib compared to BR in patients with treatment-naïve CLL/SLL. After a median follow-up of 26.2 months, PFS was significantly prolonged with zanubrutinib vs. BR (HR, 0.42; p < 0.0001) [15]. At EHA 2022, Ghia et al. presented health-related quality of life data from baseline through week 24 in patients without deletion 17p in cohort 1 who received either zanubrutinib (n = 241) or BR (n = 238) [16]. Patient-reported outcomes were collected using the EORTC QLQ-C30 questionnaire and the EuroQol EQ-5D 5-level visual analog scale.
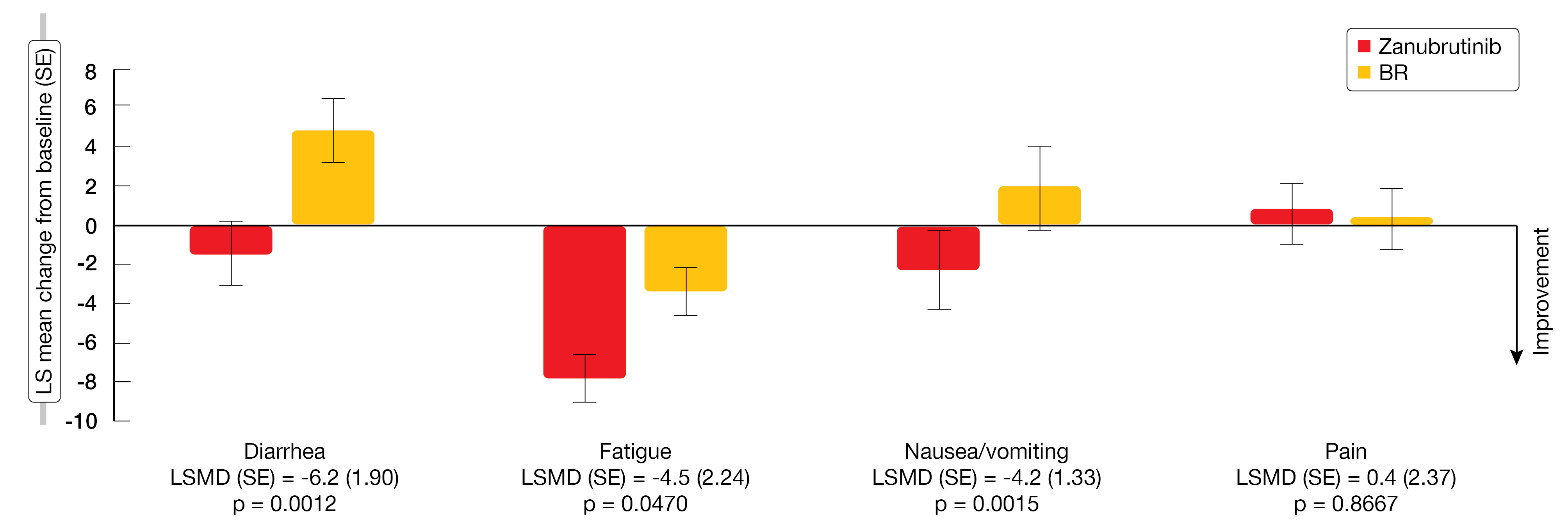
By week 12, patients in the experimental arm were shown to experience greater improvement in global health status and in physical function and role function compared to those in the control arm. Additionally, symptoms of diarrhea, fatigue and nausea/vomiting decreased from baseline to a larger extent. This symptom reduction was also observed at week 24 (Figure 5). However, at week 12, patients who received BR experienced better outcomes with respect to pain than those treated with zanubrutinib; at week 24, the effects of the two treatments on pain were similar. The authors concluded that with improved selectivity and fewer off-target effects, zanubrutinib might improve health-related quality of life outcomes in treatment-naïve patients with CLL/SLL.
Figure 5: EORTC QLQ-C30 LS mean change from baseline in symptom scales at week 24 for zanubrutinib vs. bendamustine/rituximab
ALPINE: PROs for zanubrutinib vs. ibrutinib
Zanubrutinib was compared to ibrutinib in patients with relapsed/refractory CLL/SLL in the international, open-label, randomized, phase III ALPINE trial. The interim analysis of the first 415 patients showed superiority of zanubrutinib over ibrutinib in terms of ORR, 12-month PFS, OS, and improved tolerability [17].
Health-related quality of life data based on the EORTC QLQ-C30 questionnaire and the EuroQol EQ-5D 5-level visual analog scale demonstrated greater improvements from baseline in global health status in the zanubrutinib arm compared to the ibrutinib arm by cycle 7 [18]. This also applied to physical functioning and role functioning. Moreover, zanubrutinib-treated patients experienced greater reductions in diarrhea, fatigue, nausea/vomiting, and pain. By cycle 13, findings for physical functioning and role functioning continued to favor zanubrutinib, which also applied to symptom scores for diarrhea and pain, while the two arms were comparable regarding improvements in fatigue and nausea/vomiting. With respect to the EuroQol EQ-5D 5-level visual analog scale, the analysis suggested similar patterns of improvement from baseline with zanubrutinib and ibrutinib up to cycle 13.
In their conclusion, the scientists pointed out that the more pronounced improvements of health-related quality of life in cycle 7, i.e. 6 months after the initiation of therapy, indicate that treatment with zanubrutinib could potentially alleviate disease burden earlier than ibrutinib in this patient population. The results align with the interim data obtained in ALPINE that showed lower rates of AEs such as atrial fibrillation, major bleeding, and AEs leading to discontinuation or death in patients treated with zanubrutinib vs. ibrutinib [16]. However, further analyses are warranted to assess the relationships between the health-related quality of life results and AEs as well as other clinical endpoints in this patient population.
REFERENCES
- Eichhorst B et al., A randomized phase III study of venetoclax-based time-limited combination treatments vs. standard chemoimmunotherapy in frontline chronic lymphocytic leukemia of fit patients: first co-primary endpoint analysis of the international intergroup GAIA (CLL13) trial. ASH 2021, abstract 71
- Eichhorst B et al., Time-limited venetoclax-obinutuzumab ± ibrutinib is superior to chemoimmunotherapy in frontline chronic lymphocytic leukemia: PFS co-primary endpoint of the randomized phase 3 GAIA/CLL13 trial. EHA 2022, LBA2365
- Al-Sawaf O et al., Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 5-year results of the randomized CLL 14 study. EHA 2022, S148
- Hillmen P et al., Ibrutinib plus rituximab is superior to FCR in previously untreated CLL: results of the phase III NCRI FLAIR trial. ASH 2021, abstract 642
- Hillmen P et al., The combination of ibrutinib plus venetoclax results in a high rate of MRD negativity in previously untreated CLL: the results of the planned interim analysis of the phase III NCRI FLAIR trial. EHA 2022, S145
- Woyach J et al., BTKC481S-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol 2017; 35(13): 1437-1443
- Burger JA et al., Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020; 34(3): 787-798
- Mato AR et al., Pirtobrutinib, a highly selective, non-covalent (reversible) BTK inhibitor in previously treated CLL/SLL: updated results from the phase 1/2 BRUIN study. EHA 2022, S147
- Roeker LE et al., Pirtobrutinib, a highly selective, non-covalent (reversible) BTK inhibitor in combination with venetoclax ± rituximab in relapsed/refractory chronic lymphocytic leukemia: results from the BRUIN phase 1b study. EHA 2022, P640
- Wierda WG et al., Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia: primary analysis results from the minimal residual disease cohort of the randomized phase II CAPTIVATE study. J Clin Oncol 2021; 39(34): 3853-3865
- Wierda WG et al., Fixed-duration ibrutinib + venetoclax for first-line treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma: 3-year follow-up from the FD Cohort of the phase 2 CAPTIVATE study. J Clin Oncol 40, 2022 (suppl 16; abstr 7519)
- Sharman JP et al., Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 2020; 395: 1278-1291
- Sharman JP et al., Acalabrutinib ± obinutuzumab vs obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: 5-year follow-up of ELEVATE-TN. EHA 2022, P666
- Davids MS et al., Long-term efficacy of acalabrutinib-based regimens in patients with chronic lymphocytic leukemia and higher-risk genomic features: pooled analysis of clinical trial data. EHA 2022, P667
- Tam CS et al., SEQUOIA: results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood 2021; 138: 396
- Ghia P et al., Patient-reported outcomes from a phase 3 randomized study of zanubrutinib vs. bendamustine plus rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic lymphoma. EHA 2022, P662
- Hillmen P et al., First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. EHA 2021, LB1900
- Hillmen P et al., Health-related quality of life outcomes associated with zanubrutinib vs. ibrutinib monotherapy in patients with relapsed/refractory CLL/SLL: results from the randomized phase 3 ALPINE trial. EHA 2022, P663
© 2022 Springer-Verlag GmbH, Impressum