Bruton’s tyrosine kinase inhibition in CLL: present and future
In the treatment of chronic lymphocytic leukemia (CLL), targeted drugs are continuously gaining ground. BTK-inhibitor–based treatment provides favorable results across different lines and settings including high-risk genomics, with next-generation agents offering improved efficacy and safety. At the ESH 2nd Translational Research Conference on CLL held on 17-20 March 2022, experts discussed preclinical and clinical observations.
A pharmacologist’s view
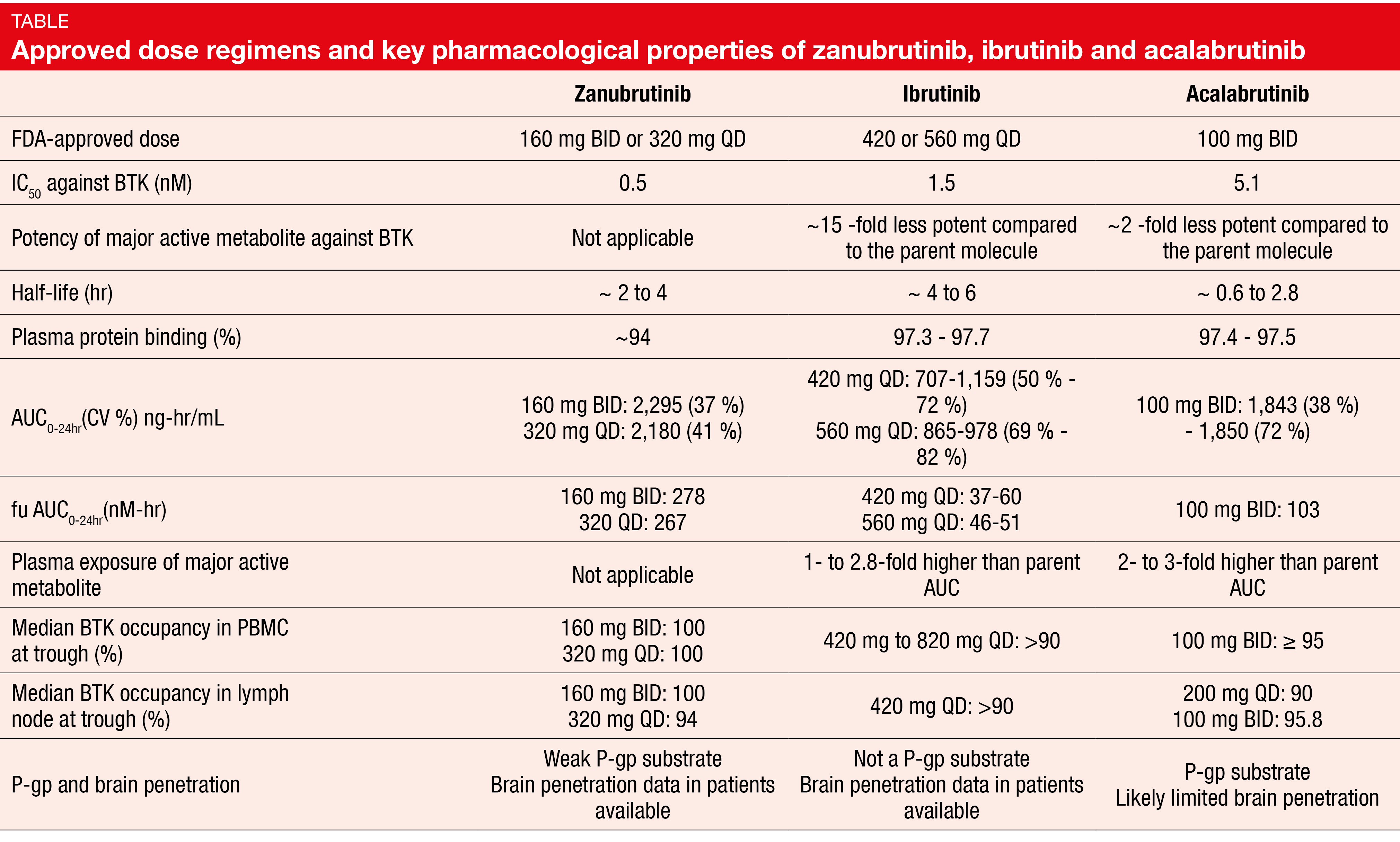
BTK inhibitors have been important game changers in the management of B-cell lymphoma in general and B-CLL and small lymphocytic lymphoma in particular. Federico Pea, MD, University of Bologna, Italy, discussed differences between the irreversible BTK inhibitors ibrutinib, acalabrutinib and zanubrutinib. The second-generation drugs acalabrutinib and zanubrutinib demonstrated higher kinase selectivity than the first-in-class agent ibrutinib, which implies an improved efficacy-toxicity ratio [1]. Zanubrutinib offers several advantages over the other inhibitors (Table). The absence of an active metabolite makes its pharmacologic behavior more predictable; lower plasma protein binding (94 %) compared to ibrutinib and acalabrutinib (97 % each) leads to higher levels of the biologically active moiety. For median BTK occupancy in peripheral blood mononuclear cells and lymph nodes at trough levels, the activity was highest with zanubrutinib.
CYP3A is the major enzyme involved in the elimination of all three BTK inhibitors. Whereas strong CYP3A inhibitors should be avoided in patients receiving ibrutinib and acalabrutinib, zanubrutinib can be prescribed as comedication at a lower dose of 80 mg QD. No restrictions limit the intake of ibrutinib and zanubrutinib in the context of acid-reducing treatment including proton pump inhibitors (PPIs), while it is recommended to avoid PPIs in patients on acalabrutinib. The labels caution against the use of ibrutinib and acalabrutinib in the presence of severe hepatic impairment; here, zanubrutinib can be co-administered at a dose of 80 mg BID.
fu, fraction of unbound drug in plasma; PBMC, peripheral blood mononuclear cell; P-gp, permeability glycoprotein
Diverging toxicity profiles
Ibrutinib has several notable toxicities including atrial fibrillation (AF), bleeding, and infections. Data reported for zanubrutinib and acalabrutinib suggest that these ibrutinib-related adverse events (AEs) do not represent a BTK inhibitor class effect but are mainly due to off-target effects as ibrutinib blocks kinases such as TEC, HER2 and HER4 [1]. The selectivity of zanubrutinib for these kinases is in the safe range, as is the selectivity of acalabrutinib for TEC and HER2. Studies demonstrated a markedly increased incidence of thrombocytopenia with ibrutinib compared to zanubrutinib and acalabrutinib [2]. Despite a higher neutropenia rate with zanubrutinib than with ibrutinib in the ASPEN study, the infection rates did not differ [3].
In their review, Makita et al. noted that next-generation BTK inhibition appears favorable among patients with comorbidities including heart disease and/or use of anticoagulants [4]. Even at toxic doses, zanubrutinib did not elicit QTc prolongation in healthy subjects [5]. Ibrutinib, but not zanubrutinib, was shown to induce platelet receptor shedding of the GPIb-IX-V complex and integrin aIIbβ3 in mice and humans, thus affecting platelet aggregation and clot retraction [6].
First-line results: ELEVATE-TN and SEQUOIA
In the first-line setting, treatment is shifting towards the use of targeted agents based on randomized clinical studies. The two paradigms that are primarily being explored include continuous monotherapy, particularly with BTK inhibitors, and fixed-duration combination therapy. Robust data support the use of ibrutinib, although the next generation of BTK inhibitors that offers improved tolerability with similar efficacy is gaining ground.
Othman Al-Sawaf, MD, University Hospital of Cologne, Germany, referred to the open-label, phase III ELEVATE-TN trial that assessed continuous acalabrutinib ± obinutuzumab in older, less fit patients [7]. Compared to fixed-duration obinutuzumab/chlorambucil, the risk of progression or death was reduced by 90 % and 80 % with acalabrutinib/obinutuzumab and acalabrutinib monotherapy, respectively (p < 0.0001 each). ELEVATE-TN was the first study to demonstrate the efficacy of second-generation BTK inhibition in an elderly and unfit population.
Similarly, zanubrutinib proved active in the SEQUOIA trial conducted in patients who were either ≥ 65 years of age or unsuitable for standard chemoimmunotherapy. Cohort 1 compared continuous zanubrutinib with bendamustine/rituximab (BR) for six cycles in a randomized manner. Patients in Cohorts 2 and 3 had del(17p) and were treated with zanubrutinib alone and zanubrutinib/venetoclax, respectively. The findings for Cohort 1 confirmed the superiority of zanubrutinib over BR, showing a 58 % reduction in the risk of progression (p < 0.0001) [8]. Cohort 2 fared well with zanubrutinib monotherapy; at 2 years, almost 90 % of patients were progression-free. Preliminary results for Cohort 3 indicated that zanubrutinib/venetoclax is well tolerated, with the vast majority of patients responding to the treatment [9].
CLL14
In terms of limited-duration strategies, randomized data comparing targeted drug combinations to other targeted combinations or single-agent chemotherapy are still scarce. The CLL14 study established improved progression-free survival (PFS) with venetoclax/obinutuzumab vs. chlorambucil/obinutuzumab in elderly, unfit patients (HR, 0.33; p < 0.0001) [10]. Minimal residual disease negativity turned out to be essential for the treatment success as it translated into PFS benefits regardless of clinical responses at the end of treatment [11].
Based on these observations, BTK and BCL2 inhibitors are deemed important CLL therapeutics independent of patient fitness or genetic risk [12]. However, single-agent chemotherapy followed by salvage with targeted therapies might still be an option, particularly in resource-limited settings. Potential future strategies include fixed-duration double and triple combinations that are currently being explored in clinical studies.
Relapsed/refractory disease: ASCEND and ELEVATE-RR
In similar vein, targeted agents represent an indisputable treatment standard in the setting of relapsed/refractory CLL. The ESMO guidelines recommend molecularly targeted therapies with BTK inhibitors and venetoclax/rituximab regimens in all relapsing/refractory patients with high-risk genetic setup or short (< 36 months) remission duration [13]. Repeating the front-line treatment is only an option in patients who had experienced long remissions, as Wojciech Jurczak, MD, PhD, Memorial Maria Skłodowska Curie National Research Institute of Oncology, Krakow, Poland, pointed out.
Randomized phase III trials illustrate the relevance of second-generation BTK inhibitors in the relapsed/refractory setup. ASCEND evaluated acalabrutinib versus the two standard-of-care regimens idelalisib/rituximab (IdR) and BR [14]. Most patients in the control arm received IdR, which made ASCEND the first study to compare a BTK inhibitor and a PI3K inhibitor in a randomized manner. Indeed, patients treated with acalabrutinib fared significantly better regarding PFS than those receiving IdR (HR, 0.29) or BR (HR, 0.36). PFS benefits were attained regardless of genomic characteristics, which underscores the efficacy of second-generation BTK inhibitors in the high-risk setting.
High-risk patients with del(17p) or del(11q) were included in the ELEVATE-RR trial assessing acalabrutinib versus ibrutinib [15]. As intended, the study demonstrated non-inferiority of the second-generation agent with respect to PFS (HR, 1.00). At the same time, the rates of AF/atrial flutter were significantly lower in the experimental arm; this also applied to rates of grade ≥ 3 AEs as well as treatment discontinuations and deaths due to AEs.
ALPINE and beyond
The ALPINE study investigated zanubrutinib compared to ibrutinib in all relapsed/refractory patients, including 20% with del(17p) and/or mutant TP53 [16]. Zanubrutinib treatment induced a superior overall response rate (78.3 % vs. 62.5 %). An even greater difference was observed in patients with del(17p) (83.3 % vs. 53.8 %). AF/atrial flutter occurred significantly less frequently in the experimental arm, and the rate of AEs leading to treatment discontinuation was almost halved (7.8 % vs. 13.0 %).
Third-generation BTK inhibitors such as pirtobrutinib can provide effective treatment even in BTK-pretreated patients, as was demonstrated by the BRUIN study [17]. Here, 68 % responded irrespective of the reason for their previous BTK inhibitor discontinuation (i.e., progression or toxicity). Therefore, according to Dr. Jurczak, physicians should not hesitate to put patients on BTK inhibition as soon as possible. Alternative options include venetoclax/rituximab and BTK/BCL2 inhibitor combinations. Several of these combination regimens are currently being investigated in clinical studies.
This is an independent report that was written without any interference from the pharmaceutical industry. Data derived from peer-reviewed scientific publications of each individual BTK inhibitor and not from head-to-head comparative studies. Results should be interpreted with caution because of differences in study designs, populations, and standards of care; conclusions cannot be drawn about comparative efficacy and safety.
REFERENCES
- Tam CS et al., Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Rev Clin Pharmacol 2021; 14(11): 1329-1344
- Sawalha Y et al., Evaluating the therapeutic potential of zanubrutinib in the treatment of relapsed/refractory mantle cell lymphoma: evidence to date. Onco Targets Ther 2020; 13: 6573-6581
- Lim KJC et al., Zanubrutinib for the treatment of Waldenström Macroglobulinemia. Expert Rev Hematol 2020; 13(12): 1303-1310
- Makita S et al., Safety considerations with targeted therapy drugs for B-cell non-Hodgkin lymphoma. Expert Opin Drug Saf 2020; 19(9): 1105-1120
- Mu S et al., No QTc prolongation with zanubrutinib: results of concentration-QTc analysis from a thorough QT study in healthy subjects. Clin Transl Sci 2020; 13(5): 923-931
- Dobie G et al., Ibrutinib, but not zanubrutinib, induces platelet receptor shedding of GPIb-IX-V complex and integrin αIIbβ3 in mice and humans. Blood Adv 2019; 3(24): 4298-4311
- Sharman JP et al., Phase 3 study of acalabrutinib combined with obinutuzumab or alone vs O plus chlorambucil in patients with treatment-naïve chronic lymphocytic leukemia, ASH 2019, abstract 31
- Tam CS et al., SEQUOIA: results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab in patients with treatment-naïve chronic lymphocytic leukemia/small lymphocytic leukemia. ASH 2021, abstract 396
- Tedeschi A et al., Zanubrutinib in combination with venetoclax for patients with treatment-naïve chronic lymphocytic leukemia or small lymphocytic lymphoma with del(17p): early results from Arm D of the SEQUIOA (BGB-3111-304) trial. ASH 2021, abstract 67
- Al-Sawaf O et al., Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 4-year follow-up analysis of the randomized CLL14 study. EHA 2021, abstract S146
- Al-Sawaf O et al., Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2020; 21(9): 1188-1200
- Hallek M & Al-Sawaf O, Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol 2021; 96(12): 1679-1705
- Eichhorst B et al., Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020; 32(1): 23-33
- Ghia P et al., ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 2020; 38(25): 2849-2861
- Byrd JC et al., Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol 2021; 39(31): 3441-3452
- Hillmen P et al., First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. EHA 2021, abstract LB1900
- Mato AR et al., Pirtobrutinib, a next generation, highly selective, non-covalent BTK inhibitor in previously treated CLL/SLL: updated results from the phase 1/1 BRUIN study. ASH 2021, abstract 391
© 2022 Springer-Verlag GmbH, Impressum