Established and novel chemo-free combinations in immuno-oncology
LEAP-007: pembrolizumab plus lenvatinib in first-line NSCLC
Immunotherapy was the major breakthrough in the treatment of lung cancer in the past years [1]. The PD-1 inhibitor pembrolizumab is approved for the treatment of various tumor entities including advanced or metastatic non-small cell lung cancer (a/mNSCLC) [2]. Lenvatinib, a multikinase inhibitor and antineoplastic agent that is so far approved for certain solid tumors but not NSCLC, already showed promising antitumoral effects and a manageable safety profile when combined with pembrolizumab in a phase I/II trial [3].
The double-blind phase III study LEAP-007 (NCT03829332) investigated the efficacy and safety of pembrolizumab with or without lenvatinib in adults with PD-L1-positive treatment-naïve NSCLC; first results were presented at ESMO IO congress 2021 [4]. Eligible patients (n=623) were randomized 1:1 to receive either pembrolizumab (200 mg intravenously [IV] every three weeks [Q3W)] for up to 35 cycles) plus oral lenvatinib (20 mg daily) or pembrolizumab plus placebo. Stratification factors were geographic region (East Asia versus non-East Asia), ECOG PS (0 versus 1), and PD-L1 TPS (1 - 49 % versus ≥ 50 %). The primary endpoint included progression-free survival (PFS) according to RECIST v1.1 assessed by a blind independent central review (BICR) and overall survival (OS), while secondary endpoints enclosed objective response rate (ORR), safety, quality of life (QoL) and patient-reported outcomes (PROs).
Baseline patient characteristics were well balanced between both arms. Median age was 66 years, about three quarter of patients were male, one third of them were enrolled in Asia, most of them were current or former smokers, while 44 % had a PD-L1 TPS ≥ 50 % and 56 % a PD-L1 TPS 1 - 49 %. The median overall survival (OS) was 14.1 months for the combination versus 16.4 months for pembrolizumab monotherapy (HR, 1.10; p = 0.79744), while the median PFS reached 6.6 versus 4.2 months, respectively (HR, 0.78; p = 0.00624). The ORR reached 40.5 % (including 7 patients with a complete response [CR] and 38 with a partial response [PR]) for pembrolizumab plus lenvatinib versus 27.7 % (including 6 CRs and 25 PRs) for pembrolizumab alone.
After a median duration of treatment of approximately six months, pembrolizumab plus lenvatinib was associated with higher rates of grade 3-5 treatment-related adverse events (TRAEs) (57.9 vs 24.4 %), as well as AEs leading to discontinuation or death, compared with pembrolizumab alone. The most common grade ≥ 3 experienced with the combined therapy were hypertension, proteinuria, or diarrhea. No new safety signals were reported.
Based on the prespecified analysis provided to the independent data monitoring committee (DMC), the benefit/risk profile for pembrolizumab plus lenvatinib was not considered favorable versus pembrolizumab alone in patients with mNSCLC presenting a PD-L1 TPS ≥1%. Thus, treatment with pembrolizumab plus lenvatinib was discontinued, but patients were allowed to continue on open-label pembrolizumab monotherapy for up to 35 cycles.
Further phase III studies evaluating pembrolizumab plus lenvatinib in NSCLC patients are ongoing. The authors concluded that pembrolizumab monotherapy remains a standard of care for first-line metastatic NSCLC with PD-L1 TPS ≥ 1 % whose tumors are not presenting with EGFR/ALK alterations.
Novel surufatinib combined with toripalimab in solid tumors
Surufatinib, a novel angio-immuno kinase inhibitor, selectively targets vascular endothelial growth factor receptor (VEGFR) 1, 2, and 3, fibroblast growth factor receptor type 1 (FGFR1) and colony stimulating factor-1 receptor (CSF-1R). In December 2020, this small molecule received its first approval in China for the treatment of late-stage, well-differentiated, extra-pancreatic neuroendocrine tumors (NET). A U.S. FDA submission for surufatinib has been filed and a submission to the EMA is planned for pancreatic and extra-pancreatic NET, too [5]. Surufatinib, which was evaluated in a dose-escalating phase I trial in combination with the humanized IgG4 PD-1 antibody toripalimab in patients with advanced solid tumors, showed encouraging antitumor activity (NCT03879057) [6] .
Results of an ongoing multicenter phase II trial investigating the efficacy and safety of surufatinib (250 mg per oral (PO) once daily) plus toripalimab (240 mg IV Q3W) in patients with unresectable or metastatic advanced solid tumors was presented at ESMO IO 2021 (NCT04169672) [7]. At the time of data cut-off (August 1, 2021), 62 patients were assigned in this open-label study into three cohorts of patients with advanced neuroendocrine carcinoma (NEC, n=21), gastric or gastroesophageal junction adenocarcinoma (G/GEJ, n=21), or advanced esophageal squamous cell carcinoma (ESCC, n=20) who progressed after 1L systemic chemotherapy. The median age was 60 years in the NEC and ESCC cohort and 58 years in the G/GEJ group. The number of male patients was slightly higher in the G/GEJ cohort than in the NEC and ESCC groups (81.0 %, 71.4 % and 70.0 %, respectively). Most of the patients had one previous anticancer therapy; approximatively 10 % of participants in the G/GEJ and ESCC cohorts had two or more prior lines of treatment. PD-L1 positivity according to low CPS (≥ 1 to < 10) was found in 61.9 %, 47.6 %, and 45.0 % of patients in the NEC, G/GEJ and ESCC cohorts, and PD-L1 high CPS (≥ 10 - < 50) was detected in 19.0 %, 28.6 %, and 35.0 % of subjects in the NEC, G/GEJ and ESCC groups, respectively.
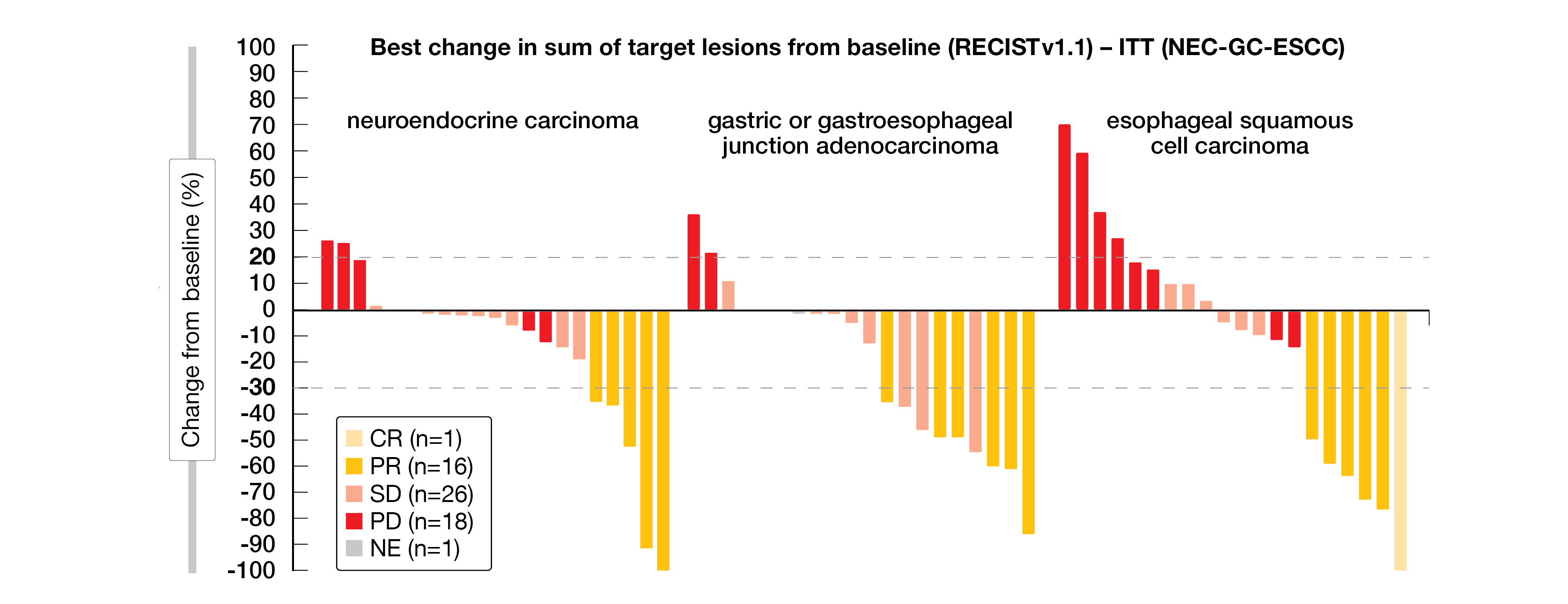
In the NEC cohort patients reached a confirmed ORR – the primary endpoint – of 23.8 %, with 5 patients with a PR, ten with a stable disease (SD) and six with a progressive disease (PD). A similar ORR of 30.0 % was reached in both G/GEJ (6 PRs, 10 SDs, 3 PDs) and ESCC cohorts (5 PRs, 6 SDs, 8 PDs) (Figure 1). One patient in the ESCC cohort had a CR. The duration of response (DoR) was 4.11 and 4.28 in the NEC and G/GEJ cohort, and not evaluable in the ESCC group. The median PFS (mPFS) – secondary endpoint – was 4.14, 4.11, and 2.73 months in the NEC, G/GEJ, and ESCC cohorts, while the mOS was 10.87, not reached, and 9.72 months, respectively.
Surufatinib in combination with toripalimab showed a manageable toxicity without any new safety signals. In total, 56.5 % of the patients in the overall group (n=62) suffered from treatment-emerged adverse events (TEAEs) of grade three or higher. Hypertension (8.1 %) as well as anemia, decreased white blood cell- and neutrophil count, and malignant neoplasm progression (6.5 % each) were the most common TRAEs.
Surufatinib in combination with toripalimab exhibited promising efficacy in patients with advanced NEC, G/GEJ and ESCC. To confirm these findings, a multicenter phase III trial in advanced NEC patients is currently ongoing in China (NCT05015621).
Figure 1: Waterfall plot of tumor response in the three cohorts.
Sintilimab plus anlotinib in second- or later line ED-SCLC therapy
Small cell lung cancer (SCLC) is still a poorly understood, aggressive disease with high relapse and mortality rates. Recently, immune checkpoint inhibitors (ICI) in combination with front-line chemotherapy significantly improved disease responses and changed the therapeutic algorithm of extensive-disease (ED)-SCLC in the first-line setting [8]. Nevertheless, treatment options for second- or later lines are limited [9].
At this year’s ESMO IO meeting, Ma et al. presented results from a phase II single-arm, open-label clinical trial; this study evaluated anlotinib – a novel receptor tyrosine kinase inhibitor (RTKi) targeting VEGFR 2 and 3, FGFR 1–4, platelet-derived growth factor receptor (PDGFR) α and β, c-KIT and RET – in combination with sintilimab – an anti-PD-1 inhibitor – in 26 patients with recurrent or ED-SCLC, who had been pretreated with at least one platinum-based chemotherapy regime [10]. Eligible patients (median age of 57 years, 76.9 % male, 23.1 % with second-line therapy, 61.6 % with stage IVb) received anlotinib (12 mg PO on Day 1-14 Q3W) combined with sintilimab (200 mg IV on D1 Q3W). After a median follow-up of nine months, the primary endpoint PFS reached 5.8 months (95 % CI, 2.8-9.4) and the mOS was 11.4 months (95 % CI, 6.2-NR). The ORR reached 41.7 %, including two patients with a CR and eight patients with a PR, and the disease control rate (DCR) was 87.5 %.
In total, all patients had TRAEs, the most common ones being thyroid dysfunction (42.3 %), hypoproteinemia and anemia (34.6 % each). Lymphocytopenia (11.5 %) and hypoproteinemia (3.8 %) were the most frequent grade 3-4 TRAEs. In three patients TRAEs led to drug discontinuation.
Given the promising efficacy and an acceptable toxicity, sintilimab in combination with anlotinib represents a potential second-line or later therapy in pretreated patients with ED-SCLC. This single center-study is currently ongoing for recruitment in China.
Pembrolizumab plus olaratumab in pretreated STS patients
Soft-tissue sarcoma (STS) is a heterogeneous group of tumors that develop from mesenchymal tissue; affected patients have a short life expectancy of approximately twelve to 18 months [11]. In a phase II study, the anti-PDGFRα antibody olaratumab in combination with doxorubicin showed an improved survival outcome in patients with STS (NCT01185964) which could not be confirmed in a subsequent phase III trial (NCT02451943). On the other hand, pembrolizumab, an anti-PD-1 antibody, has shown clinical activity in some histological subtypes of STS [12]. The addition of olaratumab to pembrolizumab might thus induce a change of the tumor microenvironment allowing pembrolizumab to elicit a more robust immune response.
An open-label phase Ia/b study assessed the safety and efficacy of the addition of pembrolizumab (200 mg IV on Day 1 of a 21-day cycle for up to 35 cycles) to olaratumab during a phase Ia (dose escalation, 15 mg/kg IV starting dose or 20 mg/kg IV escalated dose on Day 1 and 8) and a phase Ib (dose-expansion with the recommended dose of 20 mg/kg IV) study in patients with unresectable locally advanced or metastatic STS, not amenable to curative treatment and after failure of standard therapies (NCT03126591). Patients with brain metastasis were not eligible for enrollment in this trial. The primary objectives were safety and tolerability, while secondary objectives included the ORR, DCR, DoR, PFS and OS per RECIST v1.1. Study results were recently presented at ESMO IO 2021 meeting [13].
Most patients were female with an age < 65 years, and two patients hat PD-L1-positive tumors. All patients in phase 1a and > 90 % in phase 1b had received prior systemic therapy with 10 patients in phase Ia (n=13) and 13 patients in phase Ib (n=28) experienced ≥ three prior lines of therapy while ten subjects received prior olaratumab. The majority of patients in phase Ib had leiomyosarcoma (35.7 %), followed by undifferentiated pleomorphic sarcoma (10.7 %), rhabdomyosarcoma (7.1 %), as well as synovial sarcoma, alveolar soft part sarcoma, and dedifferentiated liposarcoma (3.6 % each).
In phase Ib, the ORR was 21.4 %, including six patients with PR. The DCR was 53.6 % (including additional 9 patients with SD), with a mDoR of 16.2 months, while mPFS and mOS reached 2.7 and 14.8 months, respectively. To note, no response was observed in both patients presenting with PD-L1-positive tumors.
In total, 73.2 % of the safety population (n=41) had at least one TRAE, whereas 22 % experienced grade 3 or 4 TRAEs, most commonly diarrhea and anemia (4.9 % each). Treatment discontinuation due to TRAEs occurred in two patients in phase 1a (increased lipase) and two patients in phase 1b (infection and diarrhea).
Overall, the addition of olaratumab to pembrolizumab was safe and well-tolerated in patients with advanced STS. Antitumor activity was observed in 21 % of patients in the dose-expansion cohort. Further studies with a larger sample size are needed to confirm the promising antitumor effect of this combination.
Sitravatinib combined with tislelizumab in ovarian cancer
Ovarian cancer (OC) is one of the most common gynecologic cancers, the high mortality rate is mainly caused by its diagnosis in the advanced stage because of a delayed onset of symptoms, lack of proper screening and asymptomatic and secret growth of the tumor. A platinum/taxane-based chemotherapy with or without bevacizumab is the standard of care for advanced OC [14]. The ORR of the primary treatment reaches usually 60–80 % but 70 % of patients relapse within five years and drug-resistance remains a major challenge [15]. Early phase clinical studies investigating the efficacy of ICIs have shown an antitumor activity in patients with advanced OC [15]. Tislelizumab, an PD-1 antibody, is currently investigated in a broad clinical program combining various anticancer agents [16]; among them, sitravatinib, an oral spectrum-selective kinase inhibitor that potentially inhibits TAM family receptors (TYRO3, AXL, MERTK) and split family receptors (VEGFR2, KIT) [17]. An open-label, multicenter, multicohort phase Ib study (NCT03666143) evaluated the safety and efficacy of tislelizumab in combination with sitravatinib in patients with advanced solid tumors; updated results from the cohort E (anti-PD-1/PD-L1 antibody-naïve recurrent platinum-resistant epithelial OC) have been presented at ESMO IO 2021 [18]. Safety/tolerability was the primary endpoint, while the key secondary endpoints were investigator-assessed ORR, DCR, DoR and PFS. Additionally, OS, potential pharmacodynamic biomarkers, retrospective analysis of PD-L1 expression have been exploratory analyzed.
As of March 29, 2021, 63 patients were enrolled into cohort E who received sitravatinib (120 mg PO daily) plus tislelizumab (200 mg IV Q3W) of which 27 patients (42.9 %) remained on treatment. The median age of patients was 66 years, most of them were white (79.4 %) and had serous epithelial carcinoma (95.2 %) or mucinous, endometrioid and clear cell OC (1.6 % each). In these heavily pretreated patients (median of four prior regimens), 34.9 % received bevacizumab, while 42.9 % of them had a PD-L1 expression of 10 % or higher. The median follow-up was 8.9 months.
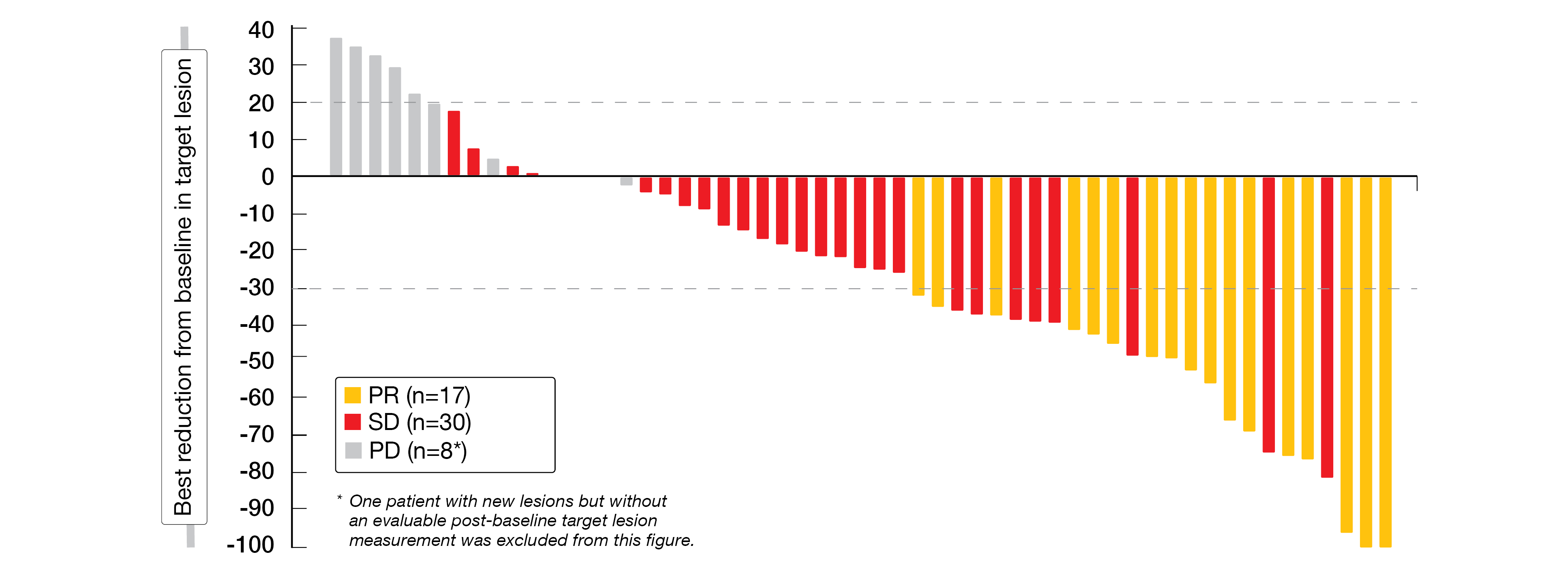
In the efficacy evaluable population (n=59), the ORR was 28.8 % (17 PRs) and the DCR 79.7 % (with 30 additional SDs); nine patients (15.3 %) had a PD (Figure 2). The overall population reached a median DoR of 5.6 months, mPFS and mOS were 4.1 and 11.8 months, respectively. After a median follow-up of 11.7 months, OS data were still immature. PD-L1 expression was not related to the clinical efficacy. Post-treatment changes of plasma VEGF, VEGFR2, and serum IP-10 were observed.
The median duration of exposure was 16 weeks for sitravatinib and 18 weeks for tislelizumab. Most patients experienced TRAEs of any grade (95.2 %) and 42.9 % grade ≥ 3 TRAEs. Frequently observed TEAEs were diarrhea (68.3 %), nausea (55.6 %) and fatigue (50.8 %), while the most common TEAEs of grade 3 or higher were hypertension (17.5 %) and fatigue (9.5 %). There were five fatal TEAEs which were unrelated to the treatment. Most patients (88.9 %) had sitravatinib dose modification.
Compared with the primary analysis (data cut-off, October 13, 2020), this two-month longer follow-up confirmed the manageable safety profile and antitumor activity of sitravatinib in combination with tislelizumab. Further investigation in this patient group is warranted.
Figure 2: Best change in target lesions related to baseline in OC patients receiving sitravatinib plus tislelizumab.
Effect of TQ-B240 plus anlotinib in NSCLC
Although ICI monotherapy has shown unclear PFS benefit and limited OS advantage compared to docetaxel alone in the second-line treatment of NSCLC [19, 20], ICIs combined to antiangiogenic agents showed a good antitumoral efficacy [21, 22]. Anlotinib, a novel multi-target anti-angiogenic RTKi, was approved in May 2018 by the China national medical products administration (NMPA) as third-line treatment for NSCLC patients after ≥ 2 lines of chemotherapy; this approval is based on the results of the phase III ALTER0303 trial, in which anlotinib significantly prolonged OS (9.6 versus 6.3 months) and PFS (5.4 versus 1.4 months) versus placebo in this patient population (NCT02388919) [23].
At ESMO IO 2021 meeting, Han et al. presented the results of a multicenter, randomized, double-blind phase Ib study investigating the safety and efficacy of TQB-2450, an investigational humanized anti-PD-L1 antibody, combined with or without anlotinib in patients with advanced NSCLC pretreated with ≥ 1 line of chemotherapy (NCT03910127) [24]. A total of 101 patients (EGFR/ALK wildtype, PD-L1 TPS unrestricted) were randomized 1:1:1 to receive either TQ-B2450 (1200 mg IV D1 Q21D) plus anlotinib (12 mg or 10 mg daily Day 1-14 of a 21-day cycle) or TQ-B2450 plus placebo; as the average daily exposed dosage of anlotinib was 10.2 mg, both TQ-B2450 plus anlotinib groups were then merged as TQ-B2450-ALTN (TQ-B2450 + anlotinib) group. The median age was 60 years in the TQ-B2450 group compared to 61.5 years in the TQ-B2450-ALTN group with 82 % and 70 % being male. Most of the patients received one previous line of treatment (70 % and 76 %), and were ever smokers (67 % and 60 %).
Nearly two third of the patients (63 %) had an adenocarcinoma in both treatment groups, followed by squamous carcinoma (27 % in the TQ-B2450 and 21 % in the TQ-B2450-ALTN group, respectively). The tumor pathology was mainly stage IV (83 % and 86 %), about one quarter of patients had brain metastases at baseline (27 % and 19 %) and half of the patients showed PD-L1 low expression (TPS 1-49 %, 50 % and 49 %).
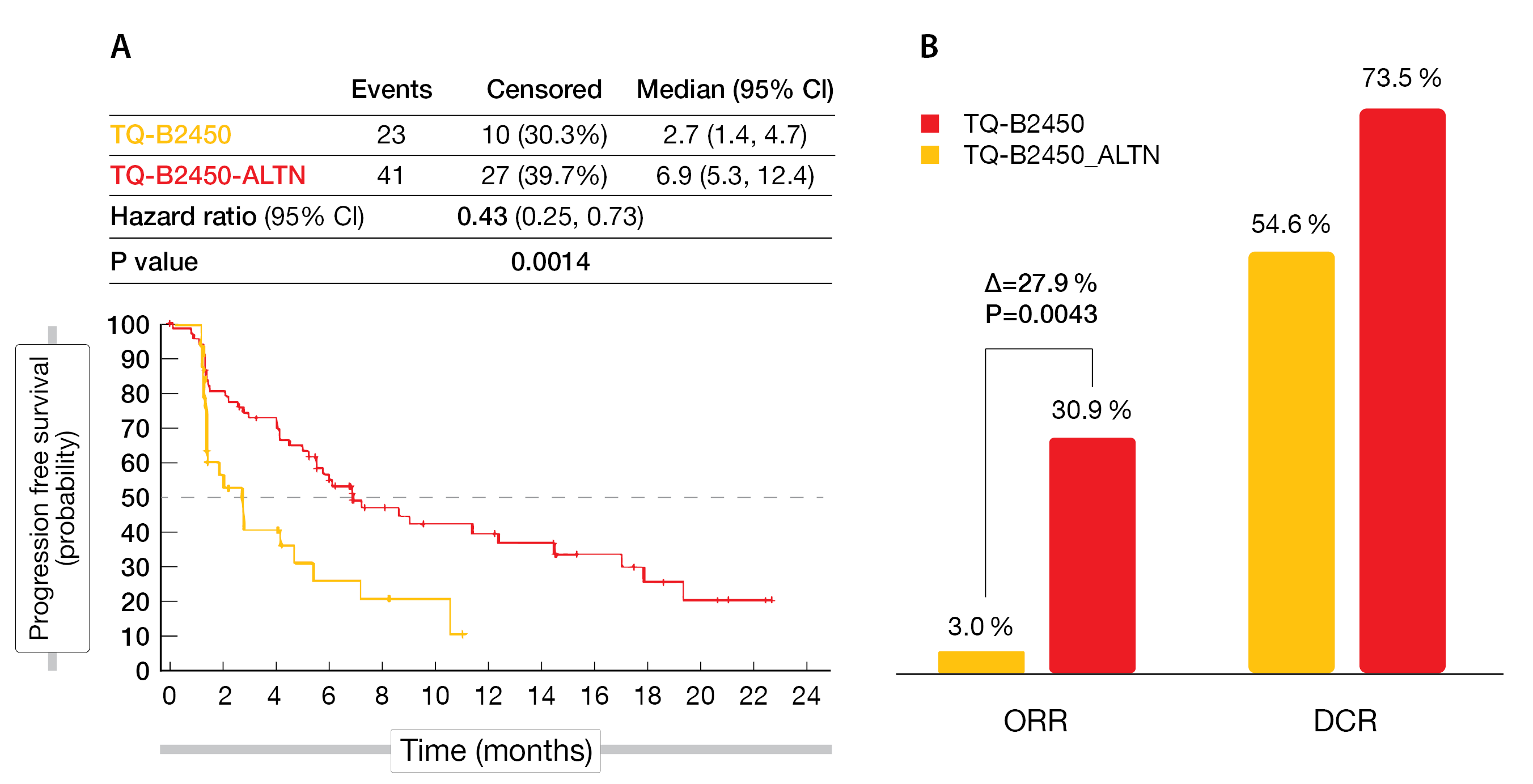
After a median follow-up of 11.1 months, TQ-B2450 plus anlotinib demonstrated a longer median PFS compared to TQ-B2450 alone (6.9 vs 2.7 months; HR, 0.43; 95 % CI, 0.25-0.73; p = 0.0014) (Figure 3A). The ORR was significantly in favor of TQ-B2450 plus anlotinib (p = 0,0043)with 30.9 % versus 3.0 %, while the DCR was 73.5 and 54.6 %, respectively (PR, 21 vs 1; SD, 29 vs 17) (Figure 3B).
Overall, 46 (67 %) patients in the combination arm and seven (21 %) in the TQ-B2450 arm experienced grade ≥3 TEAEs. The most common TRAEs in the TQ-B2450 plus anlotinib arm included hypertension (19 %) and hypertriglyceridemia (9 %). Overall, 21 % of patients discontinued the combined treatment because of TRAEs.
TQ-B2450 combined with anlotinib significantly improved PFS and ORR compared to TQ-B2450 monotherapy. Thus, this combination therapy might be a promising treatment for advanced NSCLC patients having received prior systemic therapy. A randomized phase III study of TQ-B2450 plus anlotinib versus pembrolizumab in the first-line setting of NSCLC patients with PD-L1 ≥ 1 % is ongoing (NCT04964479).
Figure 3: PFS (A) as well as ORR and DCR (B) benefit with TQ-B2450 plus anlotinib in advanced NSCLC patients.
REFERENCES
- Xiong W et al., Current Status of Immune Checkpoint Inhibitor Immunotherapy for Lung Cancer. Front Oncol 2021; 11: 704336
- Hao Z et al., Lenvatinib in Management of Solid Tumors. Oncologist 2020; 25(2): e302-e310
- Taylor MH et al., Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J Clin Oncol 2020; 38(11): 1154-1163
- Yang JC-H. et al., Pembrolizumab (Pembro) With or Without Lenvatinib (Lenva) in First-Line Metastatic NSCLC With PD-L1 TPS ≥1% (LEAP-007): A Phase 3, Randomized, Double-Blind Study. ESMO IO 2021, 120O
- Syed Y., Surufatinib: First Approval. Drugs 2021; 81(6): 727-732
- Lu M. et al., A phase I trial of surufatinib plus toripalimab in patients with advanced solid tumor. ASCO 2020, CT142
- Lu M. et al., Surufatinib plus toripalimab for 2L treatment of advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma, esophageal squamous cell carcinoma (ESCC) and neuroendocrine carcinoma (NEC): A multicenter, single-arm phase 2 study. ESMO IO 2021, 155P
- El Sayed R et al., Immunotherapy in Extensive-Stage Small Cell Lung Cancer. Curr Oncol 2021; 28(5): 4093-4108
- von Pawel J et al., Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014; 32(35): 4012-4019
- Ma S et al., Combining Sintilimab with Anlotinib as Second-Line or Later Therapy in Patients with Extensive-Disease Small Cell Lung Cancer: A Prospective, Single-Arm, Phase 2 Trial. ESMO IO 2021, 69P
- Davis EJ et al., Spotlight on olaratumab in the treatment of soft-tissue sarcoma: design, development, and place in therapy. Drug design, development and therapy 2017; 11: 3579-3587
- Tawbi HA et al., Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. The Lancet. Oncology 2017; 18(11): 1493-1501
- Schöffski P et al., Results of an open-label, phase 1a/1b study of olaratumab plus pembrolizumab in patients with unresectable, locally advanced or metastatic soft tissue sarcoma. ESMO IO 2021, 154P
- Bray F et al., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394-424
- Zhu J et al., Efficacy of PD-1/PD-L1 inhibitors in ovarian cancer: a single-arm meta-analysis. Journal of Ovarian Research 2021; 14(1): 112
- Lee A et al., Tislelizumab: First Approval. Drugs 2020; 80(6): 617-624
- Du W et al., Sitravatinib potentiates immune checkpoint blockade in refractory cancer models. JCI Insight 2018; 3(21):e124184
- Goh JC et al., Safety/tolerability and antitumor activity of sitravatinib plus tislelizumab (TIS) in patients with advanced platinum-resistant ovarian cancer (PROC). ESMO IO 2021, 153P
- Borghaei H et al., Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol 2021; 39(7): 723-733
- Herbst RS et al., Long-Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death-Ligand 1-Positive, Advanced Non-Small-Cell Lung Cancer in the KEYNOTE-010 Study. J Clin Oncol 2020; 38(14): 1580-1590
- Bang YJ et al., Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: An open-label, phase Ia/b study (JVDJ). Eur J Cancer 2020; 137: 272-284
- Herbst RS et al., Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 2019; 20(8): 1109-1123
- Cheng Y et al., Effect of anlotinib as a third- or further-line therapy in advanced non-small cell lung cancer patients with different histologic types: Subgroup analysis in the ALTER0303 trial. Cancer medicine 2020; 9(8): 2621-2630
- Han B et al., The Efficacy and Safety of TQ-B2450 alone/with Anlotinib in Previously Treated Advanced Non-Small Cell Lung Cancer (NSCLC): A Multicenter, Randomized, Double-blind, Placebo-controlled Clinical Trial. ESMO IO 2021, LBA4
© 2022 Springer-Verlag GmbH, Impressum