Immune checkpoint blockade combined with chemotherapy in solid tumors
Pooled analysis of PD-1 inhibitor versus bevacizumab in NSCLC
Non-small cell lung cancer (NSCLC) is a severe disease with poor outcomes since the majority of patients present with stage IV disease at diagnosis [1]. Approved treatment options in the first-line setting of advanced NSCLC (aNSCLC) with wildtype EGFR/ALK include bevacizumab – a VEGF targeting monoclonal antibody – and PD-1/PD-L1 inhibitors in combination with chemotherapy. Current guidelines preferentially recommend PD-1/PD-L1 antibodies over bevacizumab based on extrapolation of previous studies, since no randomized head-to-head trials exist to date [2]. With the aim of providing evidence to support clinical practice, Meng et al. presented a prospective analysis from three clinical trials at the ESMO IO congress 2021 with the aim to compare the survival benefit of PD-1 inhibitors versus bevacizumab in addition to chemotherapy for the treatment of advanced non-squamous NSCLC without targetable genetic mutations [2].
Data were pooled from three randomized trials (ORIENT-11, NCT03607539; CameL, NCT03134872; Bevacizumab trial, NCT02954172) with 466 patients who received the PD-1 inhibitors camrelizumab (also known as SHR-1210) or sintilimab in combination with pemetrexed (500 mg/m²) and platinum (cisplatin 75 mg/m² or carboplatin AUC 5 mg/mL/min), and 432 patients who received bevacizumab (15 mg/kg) plus paclitaxel (175 mg/m²) and carboplatin (AUC 6 mg/mL/min). In total, 375 patients in each arm were matched 1:1 using a propensity score matching to balance baseline characteristics of the two arms. The endpoints of this analysis included progression-free survival (PFS), overall survival (OS), and objective response rate (ORR).
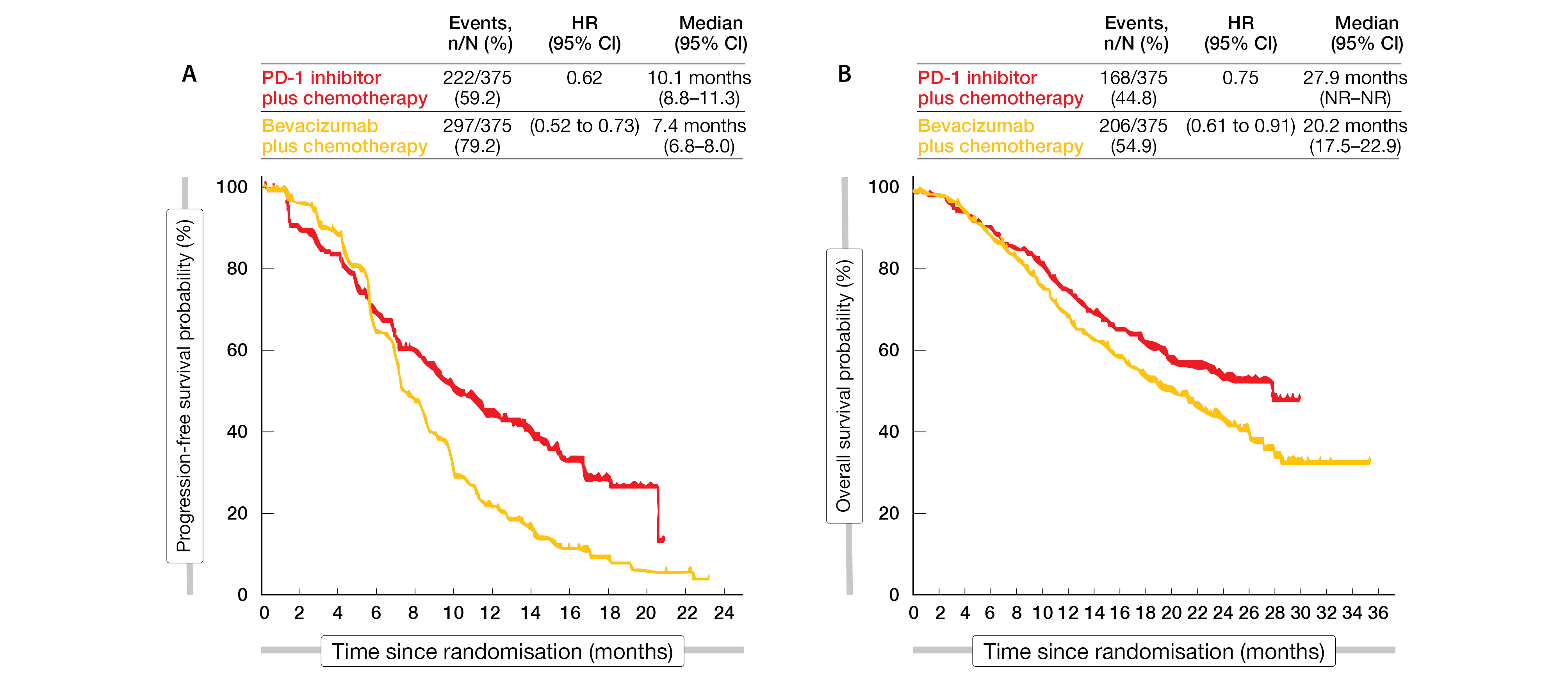
With a median follow-up of 23 months, the PD-1 inhibitors plus chemotherapy demonstrated significant longer median progression free survival (mPFS) and median overall survival (mOS) compared to bevacizumab plus chemotherapy. Indeed, the mPFS was 10.1 months in the PD-1 inhibitor arm compared with 7.4 months in the bevacizumab arm (HR, 0.62; 95 % CI, 0.52 - 0.73; p < 0.001), while the OS reached 27.9 months with PD-1 inhibitors versus 20.2 months with bevacizumab (HR, 0.75; 95 % CI, 0.61 - 0.91; p = 0.004) (Figure 1). The ORR in the PD-1 arm reached 56.8 % versus 45.1 % in bevacizumab arm.
This pooled analysis showed a significant survival benefit of PD-1 inhibitor plus chemotherapy compared to bevacizumab plus chemotherapy in patients with aNSCLC. However, an exploratory subgroup analysis indicated comparative survival outcomes in the PD-L1-negative and older patient (≥ 65 years) subgroups, thus, treatment with bevacizumab plus chemotherapy still mattered in this patient population.
Figure 1: Pooled analysis of PD-1 inhibitors versus bevacizumab: (A) PFS and (B) OS.
POSEIDON study: no ADA effects of durvalumab and tremelimumab in NSCLC
Administered therapeutic agents like immune checkpoint inhibitors (ICIs) are recognized by the immune system and may lead to the generation of anti-drug antibodies (ADAs). ADAs, by binding to the drug, can result in a reduction of the treatment activity via different mechanisms (e.g. neutralizing antibodies) or lead to increased toxicities by the generation of an immune response against the ADA-drug complex [3]. The clinical impact of ADAs upon treatment with PD-1/PD-L1 inhibitors is not well defined, and so far, data are contradictory [3, 4].
The global phase III trial POSEIDON (NCT03164616) investigated the efficacy and safety of the PD-L1 inhibitor durvalumab plus the CTLA-4 inhibitor tremelimumab. In total, 1013 patients with EGFR/ALK wild-type metastatic NSCLC were randomized 1:1:1 to durvalumab plus chemotherapy or durvalumab plus tremelimumab plus chemotherapy or chemotherapy alone as first line treatment. Main study results were published previously and showed that the combination of durvalumab plus tremelimumab plus chemotherapy significantly extended mPFS and mOS compared with chemotherapy alone [5]. Using the data set from the POSEIDON trial, Reinmuth et al. evaluated the pharmacokinetics (PK) and immunogenicity of durvalumab and tremelimumab, as well as the potential impact of ADAs on PK and safety; results of this study were presented at the ESMO IO 2021 congress [4].
PK data of 327 patients in the triplet combination arm and of 330 patients in the doublet combination were available for this analysis and showed similar profiles for durvalumab indicating that tremelimumab has no effect on durvalumab PK when administered in combination. Moreover, treatment induced-ADAs (TE-ADAs) reached 10.1 % (29/286) in the durvalumab plus tremelimumab plus chemotherapy arm and 6.7 % (19/285) in the durvalumab plus chemotherapy arm. In total, less than 3 % (8/286 and 7/285, respectively) of patients were persistently ADA-positive and about 1 % had neutralizing antibodies. Immunogenicity data for tremelimumab showed that 13.7 % (38/278) of patients had TE-ADAs, 11.2 % of ADA-evaluable patients had neutralizing antibodies, and 7.9 % (22/278) of patients were persistently ADA-positive with a low ADA titer.
Adverse Events (AEs) reported in patients positive for ADAs were similar to those reported in patients who were negative for ADAs. There were no new types of events, or events clearly suggestive or indicative of immune complex disease. Based on pooled data from prior studies, PK and immunogenicity profiles for durvalumab and tremelimumab were within expected ranges and provided no evidence that PK or safety of either treatment was adversely affected.
RATIONALE 309: tislelizumab in nasopharyngeal cancer
Nasopharyngeal cancer, a relatively rare epithelial carcinoma accounting for approximately 133,000 new cases and 80,000 deaths per year worldwide, has distinct geographical distribution and is particularly common among Asian and African populations [6, 7]. The first-line treatment with standard of care (SoC) chemotherapy has a poor prognosis with a mPFS of seven months and a mOS of 22.1 months [8, 9]. Tislelizumab – a humanized monoclonal anti-PD-1 antibody specifically designed to minimize the binding to FcγR on macrophages to abrogate the antibody dependent cellular phagocytosis – has been approved in China in December 2019 and is investigated in a broad clinical program combining various anticancer agents [10].
The randomized, double-blind, phase III trial RATIONALE 309 (NCT03924986) evaluates the efficacy and safety of tislelizumab combined with chemotherapy versus placebo plus chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal cancer (RM-NPC); results of the interim analysis were presented at ESMO IO 2021 congress [11].
A total of 263 patients were randomized 1:1 to receive either tislelizumab (200 mg intravenously [IV] every three weeks [Q3W]) plus chemotherapy (gemcitabine 1 g/m2 IV Day 1 (D1), D8 and cisplatin (80 mg/m2 D1, Q3W, 4-6 cycles) (arm A) or placebo plus chemotherapy (arm B). The primary endpoint was progression-free survival (PFS) assessed by an independent review committee (IRC), while the IRC-assessed objective response rate (ORR) and duration of response (DoR), overall survival (OS), investigator-assessed PFS, health-related quality of life (HR-QoL) and safety were secondarily evaluated. Stratification factors included gender and liver metastases. Data review was performed by an independent data monitoring committee (iDMC); cross-over to tislelizumab was allowed after disease progression or intolerable toxicity.
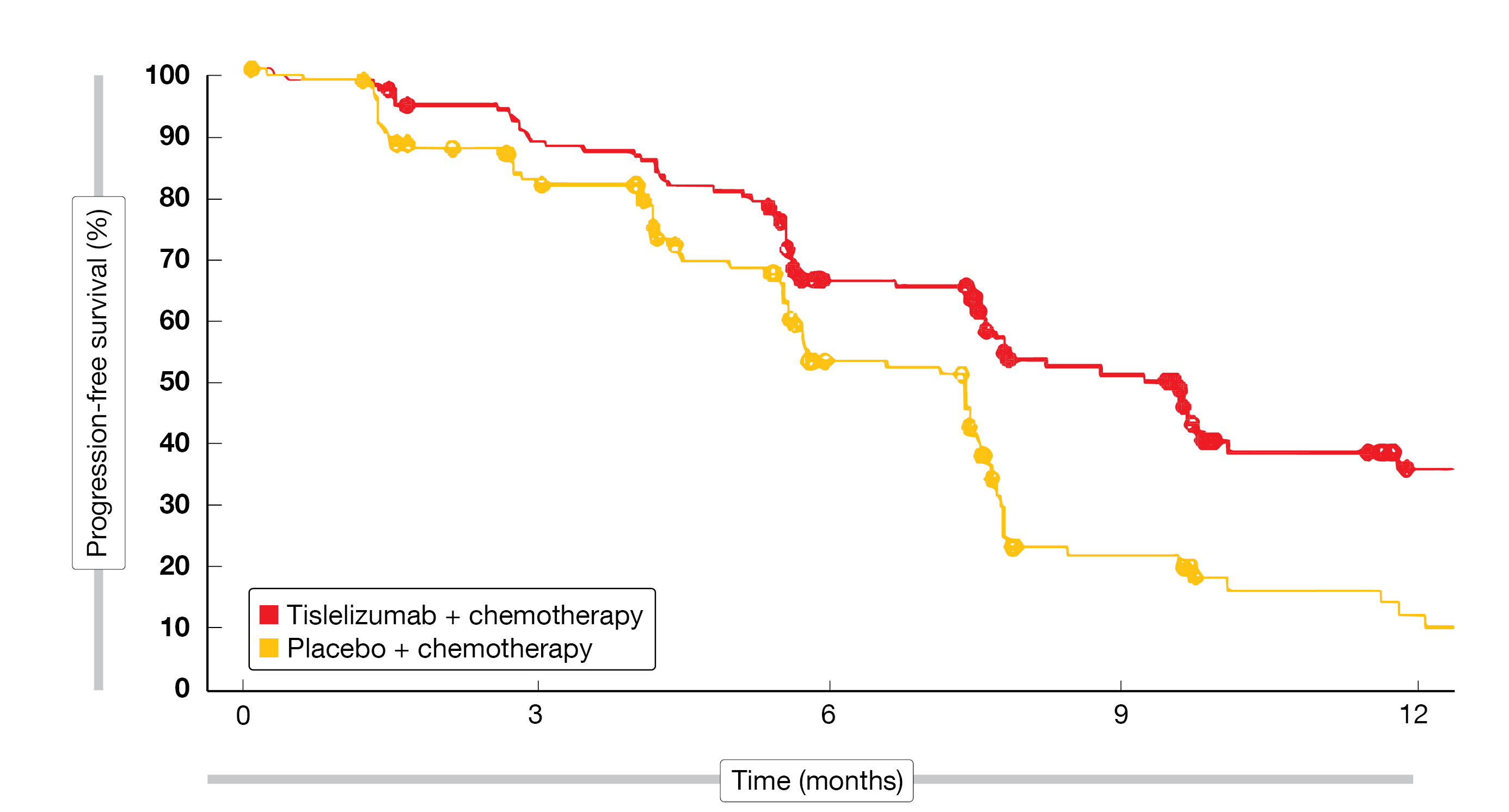
The median age of the patient population was 50 years, 78 % of the subjects were male, about half of them were never-smoker, a large majority of them had a primary metastatic disease (> 90 %) and 43 % suffered from liver metastases at baseline. In total, 80 % of patients in arm A and 72 % in arm B displayed an EBV DNA level ≥ 500 IU/ml, while 61 % and 64 %, respectively, had a PD-L1 expression ≥ 10 %. As of March 26, 2021, after a median follow-up time of ten months, the RATIONALE 309 study achieved its primary endpoint by demonstrating a statistically significant improvement of the mPFS with tislelizumab plus chemotherapy compared to chemotherapy alone (9.2 vs 7.4 months; stratified HR, 0.52; 95 % CI, 0.38 - 0.73; p < 0.0001) in the ITT population (Figure 2). The PFS rates at six, nine, and twelve months were 66.1 %, 51.0 % and 35.7 % in arm A, compared to 53.0 %, 21.6 %, and 12.2 % in arm B. Further analysis showed a significant benefit for tislelizumab plus chemotherapy in almost all subgroups. The ORR was 69.5 % versus 55.3 % in favor of tislelizumab, including 21 patients with a complete response (CR), 70 with a partial response (PR) in arm A and 9 CRs and 64 PRs in arm B. The mDoR reached 8.5 months versus 6.1 months, respectively.
The RATIONALE 309 trial showed a manageable safety profile consistent with previous reports; all patients (100 %) in arm A and 99.2 % of arm B experienced at least one treatment-emergent adverse event (TEAE) of any grade. Grade ≥ 3 TEAEs were reported in 106 patients (80.9 %) in arm A, compared to 108 patients (81.8 %) in arm B, including anemia, decreased white blood cell count, decreased neutrophil count and leukopenia. Serious TEAEs grade ≥ 3 were reported in 22.9 % of patients in arm A, compared to 26.5 % in arm B. Only 1.5 % of TEAEs in arm A and 2.3 % in arm B led to a permanent treatment discontinuation.
The authors concluded that these promising interim results support the potential of tislelizumab combined with chemotherapy as a new standard of care for the first-line treatment of RM-NPC.
Figure 2: RATIONALE 309 trial: PFS of RM-NPC patients treated with tislelizumab plus chemotherapy versus placebo plus chemotherapy.
Neoadjuvant chemotherapy plus camrelizumab in HNSCC
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide with approximately 890,000 newly diagnosed cases and a mortality rate of 51 % in 2018 [12]. Previous studies showed that neoadjuvant anti-PD-1/PD-L1 immunotherapy for resectable HNSCC was well tolerated and might be a promising therapeutic option for this patient population [13]. A phase II trial (ChiCTR1900025303) investigated the antitumoral activity and safety of the neoadjuvant anti-PD-1 antibody camrelizumab combined with chemotherapy in patients with locally advanced HNSCC; study results were presented at ESMO IO 2021 [14].
Overall, 30 eligible patients (≤ 70 years) with a histologically confirmed resectable local advanced high-risk oral, oropharyngeal, hypopharyngeal or laryngeal squamous cell carcinoma were enrolled. They received chemotherapy with either docetaxel (75 mg/m2, Day 1) plus cisplatin (75 mg/m2, Day 1) or nanoparticle albumin-bound paclitaxel (260 mg/m2, Day 1) plus cisplatin (75 mg/m2, Day 1) and camrelizumab (200 mg, Day 1) for three 21-day cycles, followed by a 10-week break, surgery, and an adjuvant radiotherapy.
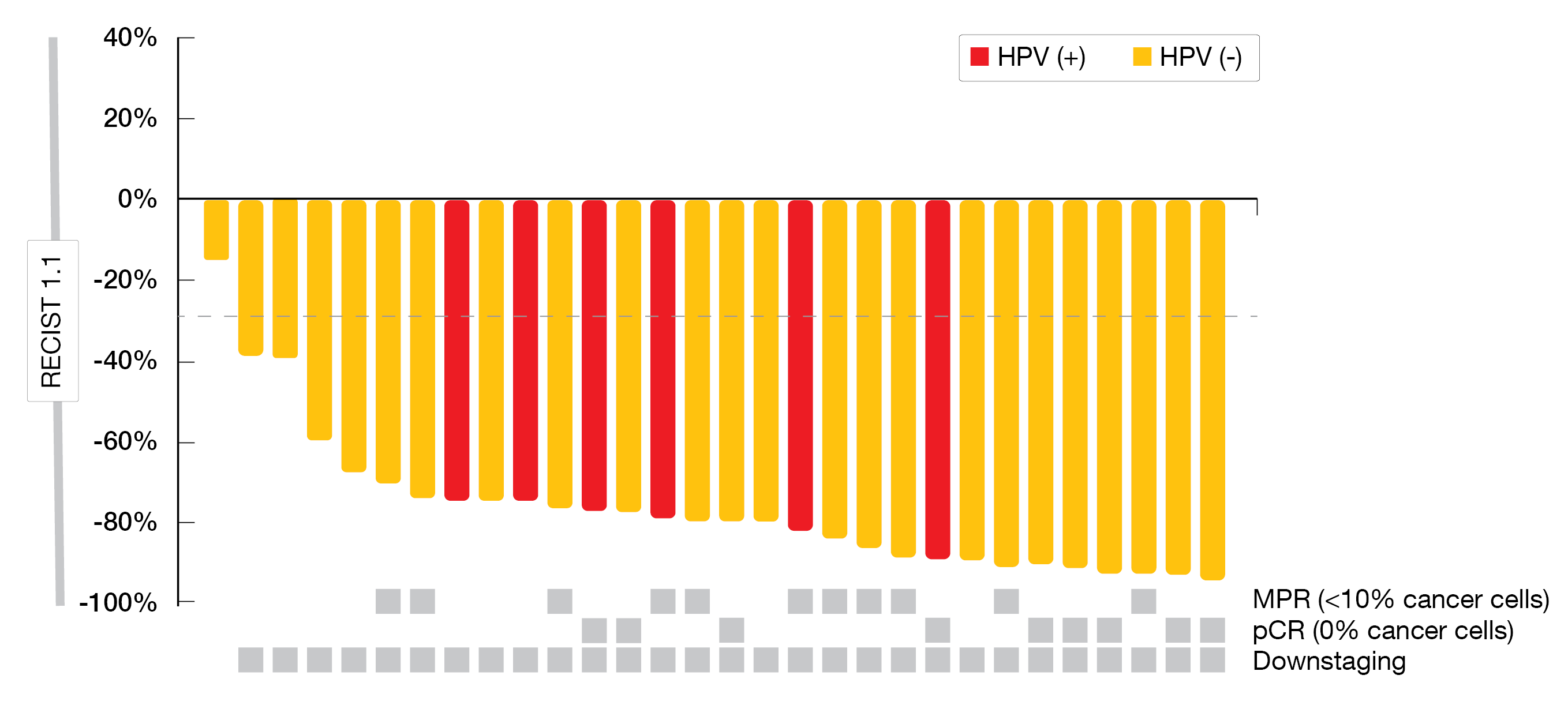
As of August 1, 2021, the median age of patients was 58 years, most of them were male (86.7 %), half of the patients were none-smokers, about one third were non-drinkers, and 20 % of patients were positive for HPV. The ORR reached 96.7 % and the disease control rate (DCR) 100 %. Three patients refused surgery, and 27 underwent surgery without delay, with an R0 resection rate of 92.6 %. The throat and hypopharyngeal function retention rate was 84.6 %. The pathological complete response (pCR) rate, which was the co-primary endpoint with safety, reached 33.3 % after a median follow-up of 10.5 months; the major pathological response rate was 74.1 % and the disease-free survival (DFS) not reached (Figure 3).
Most of the neoadjuvant TRAEs were mild. Grade 3 rash, pruritus, and thrombocytopenia were experienced by one patient each. No grade 4 or 5 TREAs were observed.
Neoadjuvant chemotherapy plus camrelizumab demonstrated a strong anticancer activity with an acceptable safety profile and might thus be considered a new treatment option for locally advanced HNSCC in the neoadjuvant setting.
Figure 3: Tumor response per RECIST v1.1 and pathological assessment according to HPV status.
1L toripalimab combined with chemotherapy in advanced thymic carcinoma
Thymic epithelial tumors (TETs) include thymomas (Ts) and thymic carcinomas (TCs); TCs, which account for approximately 15 to 20 % of TETs, represent an aggressive disease with poor prognosis [15]. TCs highly express PD-L1 and previous data indicate a promising antitumoral effect of pembrolizumab monotherapy [16, 17].
At ESMO IO 2021 meeting, Hu et al. presented the study design of an open-label, single-arm, phase II study (ChiCTR2000039155), which aims to investigate the efficacy and safety of the PD-1 inhibitor toripalimab combined with paclitaxel and carboplatin in treatment-naïve patients with advanced TCs [18]. Key eligible criteria include the following: ≥ 18 years, ECOG PS ≤ 2, histologically or cytologically confirmed Masaoka stage III or IV TC, no prior systemic anticancer therapy for advanced TCs, at least one measurable lesion per RECIST v1.1 and adequate organ function. Patients with symptomatic brain metastases or controlled symptoms for less than one month are ineligible for enrollment, as well as patients who had a prior allogeneic hematopoietic stem cell- or organ transplantation. Approximately 30 patients will be enrolled and receive toripalimab (240 mg, Day 1) in combination with paclitaxel (175 mg/m2, Day 1) and carboplatin (AUC=5, Day 1), Q3W, for six cycles. Patients whose disease did not progress will continue treatment with toripalimab (240 mg, Day 1, Q4W) until disease progression, intolerable toxicity, or withdrawal following patient’s request. Investigator-assessed PFS according to RECIST v1.1 is the primary endpoint of the study, while secondary endpoints are ORR, OS, DCR, DoR, time to response (TTR) and safety.
The enrollment into this single-center trial has started in March 2021 in Beijing, China.
Dual targeting with ociperlimab plus tislelizumab combined with cCRT in NSCLC
About 85 % of lung cancers – the most common cancer worldwide – are NSCLCs [19]. Approximately one third of patients first diagnosed with NSCLC present with locally advanced stage III disease and have a poor long-term prognosis [19]. Combined chemoimmunotherapy showed a synergistic anticancer activity in the first-line treatment of advanced NSCLC [20]; however, most patients still suffer from disease recurrence [21]. However, immunotherapy after chemoradiotherapy (cCRT) was shown to improve survival outcomes in patients with locally advanced unresectable disease [22]. Tislelizumab, a humanized IgG 4 monoclonal antibody, was designed to abolish the antibody-dependent phagocytosis, a potential mechanism of resistance to anti-PD-1 therapy [10]. The investigational humanized anti-TIGIT antibody ociperlimab binds specifically to TIGIT and blocks its interaction with the poliovirus receptor (CD155) and poliovirus receptor-related (CD112) ligands on tumor cells, resulting in activation of T-cell mediated antitumor immune response [23, 24].
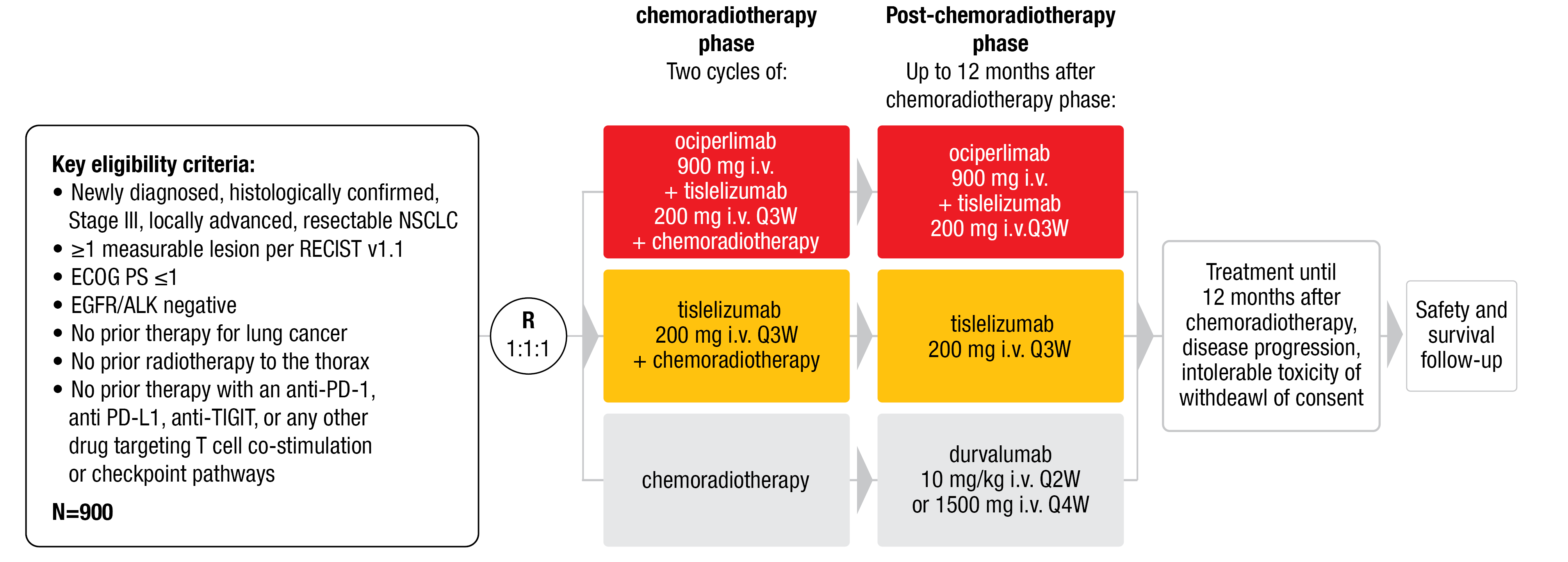
Xing et al. presented the study design (Figure 4) of an ongoing randomized phase III trial named AdvanTIG-301 (NCT04866017) at ESMO IO 2021. This multicenter, randomized, open-label study, where patients are randomized 1:1:1, is investigating the efficacy and safety of ociperlimab plus tislelizumab plus cCRT followed by ociperlimab plus tislelizumab (arm A) compared with tislelizumab plus cCRT followed by tislelizumab (arm B) or cCRT followed by durvalumab (arm C) in previously untreated, locally advanced, unresectable NSCLC [24]. Key inclusion criteria are: i) untreated, newly diagnosed, histologically confirmed, locally advanced stage III unresectable NSCLC in absence of EGFR or ALK genomic tumor aberrations; ii) ECOG PS ≤ 1; and iii) no prior therapies (listed in Figure 4). Co-primary endpoints to be assessed by an IRC according to RECIST v1.1 are PFS and complete response rate (CRR). Secondary endpoints include OS, ORR, DoR, time to death or distant metastasis, HR-QoL, safety and tolerability, serum concentrations of ociperlimab and tislelizumab, immunogenic response to ociperlimab and tislelizumab as well as the evaluation of PD-L1 and TIGIT expression. An exploratory analysis will additionally evaluate biomarkers and patient-reported outcomes (PROs).
Recruitment is currently ongoing in various study sites of the United States and Australia.
Figure 4: AdvanTIG-301 study design
REFERENCES
- Uhlig J et al., Comparison of Survival Rates After a Combination of Local Treatment and Systemic Therapy vs Systemic Therapy Alone for Treatment of Stage IV Non-Small Cell Lung Cancer. JAMA Netw Open 2019; 2(8): e199702
- Meng X et al., PD-1 inhibitor plus chemotherapy versus bevacizumab plus chemotherapy in patients with advanced non-squamous non-small-cell lung cancer: a pooled analysis of three randomized trials. ESMO IO, 2021, 62P
- Enrico D et al., Antidrug Antibodies Against Immune Checkpoint Blockers: Impairment of Drug Efficacy or Indication of Immune Activation? Clin Cancer Res 2020; 26(4): 787-792
- Reinmuth N et al., Durvalumab (D) ± tremelimumab (T) + chemotherapy (CT) in first-line metastatic NSCLC (mNSCLC): Pharmacokinetics (PK) and anti-drug antibody (ADA) data from the Phase 3 POSEIDON trial. ESMO IO 2021, 158P
- American Association for Cancer Research, Three-Drug Regimen Bests Chemo in NSCLC. Cancer Discov 2021; 11(11): Of2
- Chen YP et al., Nasopharyngeal carcinoma. Lancet 2019; 394(10192): 64-80
- Sung H et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209-249
- Hong S et al., Gemcitabine Plus Cisplatin Versus Fluorouracil Plus Cisplatin as First-Line Therapy for Recurrent or Metastatic Nasopharyngeal Carcinoma: Final Overall Survival Analysis of GEM20110714 Phase III Study. J Clin Oncol 2021; 39(29): 3273-3282
- Zhang L et al., Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016; 388(10054): 1883-1892
- Lee A et al., Tislelizumab: First Approval. Drugs 2020; 80(6): 617-624
- Yang Y et al., RATIONALE 309: A randomized, global, double-blind, Phase 3 trial of tislelizumab (TIS) vs placebo, plus gemcitabine + cisplatin (GP), as 1L treatment for recurrent/metastatic nasopharyngeal cancer (RM-NPC). ESMO IO 2021, 121O
- Johnson DE et al., Head and neck squamous cell carcinoma. Nature reviews. Disease primers 2020; 6(1): 92
- Masarwy R et al., Neoadjuvant PD-1/PD-L1 Inhibitors for Resectable Head and Neck Cancer: A Systematic Review and Meta-analysis. JAMA Otolaryngol Head Neck Surg 2021; 147(10): 871-878
- Yang K et al., Neoadjuvant chemotherapy combined with camrelizumab for locally advanced head and neck squamous cell carcinoma: a phase 2 trial. ESMO IO 2021, 145P
- Scorsetti M et al., Thymoma and thymic carcinomas. Crit Rev Oncol Hematol 2016; 99: 332-350
- Giaccone G et al., Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018; 19(3): 347-355
- Yokoyama S et al., Thymic tumors and immune checkpoint inhibitors. J Thorac Dis 2018; 10(Suppl 13): S1509-s1515
- Hu X et al., A Phase II Study of Toripalimab Combined with Paclitaxel/Carboplatin for the First-line Treatment of Advanced Thymic Carcinoma. ESMO IO 2021, 117TiP
- Tun AM et al., Checkpoint inhibitors plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Future science OA 2019; 5(9): FSO421
- Gandhi L et al., Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018; 378(22): 2078-2092
- Sui H et al., Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. J Immunol Res 2018; 2018: 6984948
- Gray JE et al., Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol 2020; 15(2): 288-293
- Harjunpää H et al., TIGIT as an emerging immune checkpoint. Clin Exp Immunol 2020; 200(2): 108-119
- Xing L et al., AdvanTIG-301: Anti-TIGIT monoclonal antibody (mAb) ociperlimab (OCI) + tislelizumab (TIS) + concurrent chemoradiotherapy (cCRT) followed by OCI + TIS or TIS + cCRT followed by TIS vs cCRT followed by durvalumab (DUR) in previously untreated, locally advanced, unresectable NSCLC. ESMO IO 2021, 167TiP
© 2022 Springer-Verlag GmbH, Impressum