Emerging therapies in solid tumors
BMS-986253 plus nivolumab and ipilimumab
Interleukin-8 (IL-8), also known as chemokine (C-X-C motif) ligand 8, is a pro-inflammatory chemokine that exerts direct pro-tumorigenic effects primarily by recruiting immunosuppressive cells into the tumor microenvironment such as neutrophils and myeloid-derived suppressor cells. IL-8 has also been shown to promote cancer progression and resistance to therapy, by inducing angiogenesis, epithelial-mesenchymal transition (EMT), and cancer stem cell (CSC) self-renewal. Moreover, elevated serum levels have been associated with a poor prognosis in patients with different solid tumors and a reduced clinical benefit from immune checkpoint inhibitors (ICIs) therapy [1, 2]. BMS-986253 is a novel fully humanized monoclonal antibody that prevents IL-8 signaling, through the C-X-C motif chemokine receptors 1 and 2, by binding free IL-8 [3]. IL-8 blockade results in the inhibition of myeloid-derived suppressor cells recruitment to the tumor environment, in improved T-cell activity, decreased tumor growth, angiogenesis, and metastasis of cancer cells. Matteo Simonelli presented updated data of the initial part of a phase I trial (NCT03400332) designed to explore dosing and safety of BMS-986253 plus nivolumab with or without ipilimumab in patients with advanced solid tumors at ESMO IO 2022 [4].
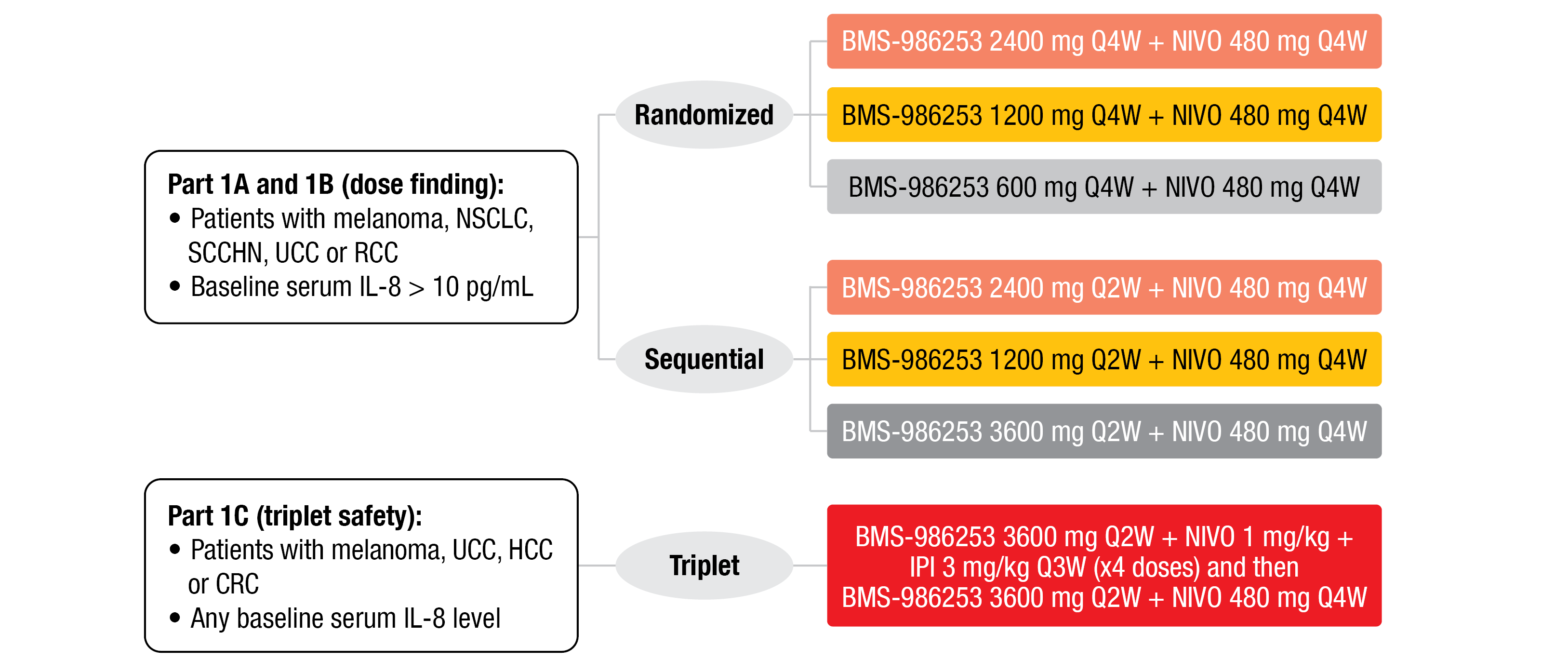
Patients recruited in the dose finding phase of the trial (part 1A and 1B) had different selected metastatic solid tumors (melanoma, NSCLC, SCCHN, UCC, or RCC), and a baseline IL-8 serum level of more than 10 pg/mL. They received different doses and schedules of BMS-986253 (2400 mg, 1200 mg, or 600 mg, every four weeks [Q4W]; 3600 mg, 2400 mg, or 1200 mg, Q2W) given in combination with nivolumab (480 mg, Q4W) (Figure 1). Patients included in the safety part (1C) had solid tumors (NSCLC not included) and any baseline IL-8 serum level. They received BMS-986253 (3600 mg, Q2W) plus four doses of nivolumab (1 mg/kg) and ipilimumab (3 mg/kg, Q3W), followed by BMS-986253 (3600 mg, Q2W) plus nivolumab (480 mg, Q4W).
As of the data cut-off date (August 4, 2022), 144 patients received the doublet combination of BMS-986253 plus nivolumab (part 1A + 1B) and 15 patients the triplet combination (part 1C). BMS-986253 plus nivolumab was well-tolerated with no dose-proportional increase in toxicity and no dose-limiting toxicities (DLTs) observed at any dose level. Since, 3600 mg BMS-986253 (Q2W) plus nivolumab showed a good tolerability, it was selected as the recommended phase II dose. In the dose expansion group (part 1A + 1B), eleven patients (8 %) experienced a grade ≥ 3 TRAEs and only two (1 %) patients discontinued due to serious TRAEs (one grade 4 infusion-related reaction and one grade 3 increase in AST/ALT). In the triplet group (part 1C), 33 % of patients experienced grade ≥ 3 TRAEs and 13 % discontinued because of TRAEs. The safety profile in this group compared favorably to that of the CheckMate 067 trial, which used the same nivolumab plus ipilimumab dosing regimen [5, 6].
In the dose exploring cohort (part 1A + 1B), six (13 %) out of 46 patients with melanoma achieved a durable response, as assessed per RECIST v1.1. All responding patients had received prior anti-PD-1 therapy and five out of six of them had received also a previous an anti-CTLA-4 therapy. Overall, 26 % remained on therapy for more than six months and 9 % for more than twelve months. In the triplet therapy cohort (part 1C), one out of six patients with melanoma obtained a partial response (PR). BMS-986253 exposure increased in a dose-dependent manner and treatment resulted in a concentration-dependent free serum IL-8 reduction that correlated with tumour-IL-8 suppression.
The authors concluded that BMS-986253 plus nivolumab with or without ipilimumab proved to be safe, while inducing a durable antitumor activity with concentration-dependent reductions in free serum IL-8 in a heterogenous patient population with advanced cancer. Furthermore, these findings support the opening of the second part of this trial, currently ongoing, which is a double-blind randomized study confronting the triplet (BMS-986253 + Nivo + Ipi) vs Nivo plus Ipi and placebo in patients with melanoma progressed on or after previous anti–PD-1 therapy.
Figure 1: Design of the phase 1 trial evaluating BMS-986253 safety evaluation and dose exploration.
IPI, ipilimumab; NIVO, nivolumab
1L tislelizumab plus lenvatinib
In hepatocellular carcinoma (HCC), that accounts for the sixth most prevalent cancer worldwide [7], emerging therapies include e.g. tislelizumab, a monoclonal antibody characterized by a high binding affinity for PD-1 and a reduced Fcγ receptor binding on macrophages [8]. The previous phase III RATIONALE-301 trial has demonstrated the non-inferiority of tislelizumab versus sorafenib as 1L monotherapy in patients with unresectable HCC (uHCC) in terms of overall survival (OS) [9].
At the ESMO IO 2022 meeting, Li Xu reported on the preliminary outcomes of a phase II trial conducted in systemic treatment-naïve patients with uHCC to assess efficacy and safety of tislelizumab plus lenvatinib, a multikinase inhibitor approved as first-line treatment in uHCC patients [10, 11].
Eligible patients had a locally advanced or metastatic uHCC, no prior systemic therapy, a BCLC stage C or B disease not amenable to or progressed after loco-regional therapy, a class A Child-Pugh score, at least one measurable lesion per RECIST v1.1, an ECOG performance status ≤ 1 and no tumor thrombus involving main trunk of portal vein or inferior vena cava. In part 1 of the study (safety run-in), patients received tislelizumab (200 mg, Q3W, IV) plus lenvatinib (body weight [BW] ≥60 kg, 12 mg; BW <60 kg, 8 mg; QD, PO). During the expansion phase (part 2), patients received tislelizumab (200 mg, Q3W, IV) plus lenvatinib (12 mg [BW ≥ 60 kg] or 8 mg [BW < 60 kg], QD, PO) until progression, unacceptable toxicity, 12-month treatment duration completion or death. The primary endpoint was ORR as assessed per RECIST v1.1 by an independent review committee (IRC), with a statistical assumption that ≥ 18 responders were needed in 60 efficacy evaluable patients to claim statistical superiority to a historical control ORR of 18.8 % per RECIST v1.1 (from lenvatinib arm of phase III REFLECT study [10]). Secondary endpoints consisted in safety and tolerability, and the ORR, DoR, DCR and PFS as assessed respectively per RECIST v1.1, mRECIST and iRECIST by IRC and investigator (except for primary endpoint).
Six patients were enrolled in the safety run-in phase and 58 patients in the expansion phase. In total, 21.9 % of the patients were still on therapy at the time of data cut-off (July 7, 2022). The median age was 52.5 years (range, 28.0-70.0) and they presented mainly with a BCLC staging of C (73.4 %) at study entry, as well as Child-Pugh score of 5 (90.6 %).
The median follow-up time was 12.5 months. Among the 62 evaluable patients for efficacy, 23 of the first 60 patients responded to the treatment. The confirmed ORR was 38.7 % (CR, 0 %; PR, 38.7 %) per RECIST v1.1 by IRC and 41.9 % (CR, 1.6 %; PR, 40.3 %) per RECIST v1.1 by investigator review, while DCR reached 90.3 % and 85.5 %, respectively. The ORR outcomes were globally similar across RECIST v1.1, mRECIST and iRECIST assessment methods. The median DoR per RECIST v1.1 was not reached, while the median PFS was 9.6 months (95 % CI, 6.8-NE) and 8.5 months (95 % CI, 5.3-NE) by IRC and investigator review, respectively. Reductions in tumor size of target lesion were observed in 74.2 % and 80.6 % of the patients, respectively.
During the safety run-in phase, no DLT was reported. Treatment-related adverse events (TRAEs) grade ≥ 3 and treatment-related serious AEs occurred in 28.1 % and 9.4 % of patients. Overall, 3.1 % of TRAEs grade ≥ 3 led to treatment discontinuation and 1.6 % to death.
This study met its endpoint of statistical superiority over a historical control (lenvatinib arm of the phase III REFLECT study [10]) in the 1L setting in uHCC patients, with a confirmed ORR of 38.7 % per RECIST v1.1 by IRC review. Tislelizumab plus lenvatinib was efficient and generally well tolerated in uHCC patients, while showing a promising mPFS.
REFERENCES
- David, J, et al., The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016; 4(3): 22.
- Schalper, KA, et al., Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nature Medicine 2020; 26(5): 688-692.
- Bilusic, M, et al., Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J ImmunoTherapy Cancer 2019; 7(1): 240.
- Simonelli, M, Anti-IL-8 BMS-986253 + nivolumab (NIVO) ± ipilimumab (IPI) in patients (pts) with advanced cancer: update of initial phase 1 results. Ann Oncol 2022; 12 (Suppl. 1): Abstract 200MO.
- Brahmer, JR, et al., Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J ImmunoTherapy Cancer 2021; 9(6): e002435.
- Wolchok, JD, et al., Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. NEJM 2017; 377(14): 1345-1356.
- Global Cancer Observatory: Cancer Today. 2022, Accessed December 2022.
- Zhang, T, et al., The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunology, Immunotherapy 2018; 67(7): 1079-1090.
- Qin, S, et al., Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol 2022; 33 (Suppl. 7): Abstract LBA36.
- Kudo, M, et al., Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet 2018; 391(10126): 1163-1173.
- Xu, L, et al., Efficacy and safety of tislelizumab (TIS) plus lenvatinib (LEN) as first-line treatment in patients (pts) with unresectable hepatocellular carcinoma (uHCC): A single-arm, multicenter, phase II trial. Ann Oncol 2022; 33 (Suppl 7): Abstract 165P.
© 2023 Springer-Verlag GmbH, Impressum
More posts
Emerging therapies in solid tumors
Emerging therapies in solid tumors BMS-986253 plus nivolumab and ipilimumab Interleuki
New strategies with PD-1/PD-L1 blockade in lung cancer
New strategies with PD-1/PD-L1 blockade in lung cancer Small-cell lung cancer (SCLC) ac
Preface – ESMO IO 2022
Preface - ESMO IO 2022 Matteo Simonelli, MD, Department of Biomedical Sciences, Humani