Immunotherapy: once more at the cutting edge of progress
PACIFIC: durvalumab in stage III NSCLC
Approximately one third of patients with non–small-cell lung cancer (NSCLC) presents with stage III, locally advanced disease. For those with good performance status and unresectable tumours, the standard of care is platinum-based doublet chemotherapy with concurrent radiotherapy. As no major advances have occurred in this setting over several years, there is a significant unmet need for novel therapeutic approaches to boost survival. Given the efficacy of checkpoint inhibitors in metastatic disease, the global, double-blind PACIFIC trial was initiated as the first randomised phase III study to evaluate immune checkpoint blockade in patients with stage III, locally advanced, unresectable NSCLC.
PACIFIC assessed the PD-L1 inhibitor durvalumab in patients who had not progressed following definitive platinum-based concomitant chemoradiation therapy of at least 2 cycles. They were randomised to either durvalumab 10 mg/kg every 2 weeks for up to 12 months (n = 476) or placebo (n = 237). This was an all-comer population without any restrictions regarding PD-L1 expression status. Half of the patients in each arm had squamous and non-squamous histology, respectively. The majority had obtained partial response (PR) or stable disease (SD) at the end of chemoradiation therapy. Progression-free survival (PFS) by blinded independent central review (BICR) using RECIST v1.1 and overall survival (OS) constituted the co-primary endpoint.
PFS difference of more than 11 months
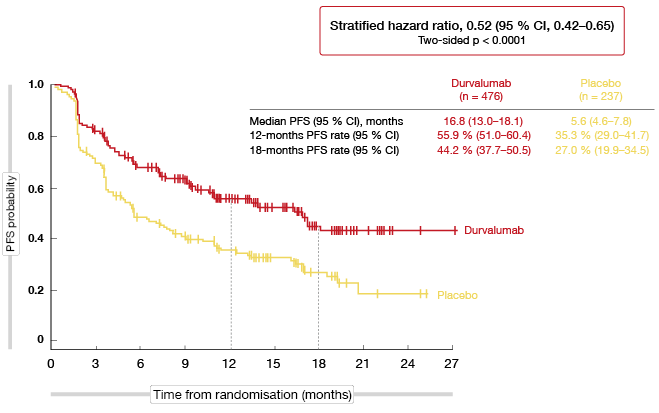
The results of the planned interim analysis for PFS presented at the ESMO 2017 Congress after a median follow-up of 14.5 months indicated that durvalumab is a promising therapeutic option in the stage III setting [1]. As compared to placebo, durvalumab demonstrated a statistically significant and robust PFS benefit with a median improvement of more than 11 months (16.8 vs. 5.6 months; HR, 0.52; p < 0.0001; Figure 1). The PFS curves started to separate during the second month after treatment initiation. All pre-specified subsets benefited from the durvalumab treatment, with a similar magnitude of benefit regardless of features such as histology, best response to chemoradiation therapy, and PD-L1 expression status.
Likewise, the objective response rate (ORR) was improved in the durvalumab arm to a clinically meaningful extent (28.4 % vs. 16.0 %; p < 0.001). This also applied to the duration of response (not reached vs. 13.8 months; HR, 0.43). Accordingly, new lesions at any site, including brain metastases, developed less frequently in the experimental arm than in the placebo arm (20.4 % vs. 32.1 %), and time to distant metastasis or death by BICR was significantly prolonged (23.2 vs. 14.6 months; HR, 0.52; p < 0.0001).
Durvalumab showed a favourable safety profile that was consistent with prior reports in more advanced disease. Cough, pneumonitis, pyrexia, pneumonia, rash and hypothyroidism counted among the most frequent adverse events (AEs). No new safety signals emerged after chemoradiation treatment. For pneumonitis/ radiation pneumonitis, the difference between the durvalumab arm and the placebo arm was small (any grade, 33.9 % vs. 24.8 %). Fifteen percent compared to 10 % of patients discontinued therapy due to AEs. Immune-related AEs of any grade were observed in 24.2 % vs. 8.1 %, with low percentages of grade 3/4 events (3.4 % vs. 2.6 %). The study remains blinded to OS, as the final analysis of OS will be performed after the target number of deaths has been reached.
Figure 1: Progression-free survival with durvalumab vs. placebo in the PACIFIC trial
Confirming atezolizumab activity in PD-L1–negative patients
The randomised OAK [2] and POPLAR [3] trials showed that treatment with the anti-PD-L1 antibody atezolizumab in the second line and beyond gives rise to clinically relevant improvements in OS versus docetaxel regardless of PD-L1 expression or histology. According to the primary analysis of OAK, median OS was 13.8 and 9.6 months for atezolizumab and docetaxel, respectively (HR, 0.73; p = 0.0003) [2]. A distinct feature of the OAK results is that atezolizumab improved OS across all of the PD-L1 expression subgroups, including patients whose PD-L1 status was negative according to the SP142 assay. In this group, the HR for OS was 0.75, thus resembling the overall HR of 0.73, and median OS was 12.6 vs. 8.9 months with atezolizumab and docetaxel, respectively.
A retrospective exploratory analysis of the OAK trial confirmed that atezolizumab provides survival benefit in all patients regardless of PD-L1 status, and demonstrated improved OS in those with PD-L1-negative tumours irrespective of the assay utilised [4]. To this end, the investigators compared the two FDA-approved PD-L1 immunohistochemistry (IHC) diagnostic assays SP142 and 22C3. Tumours of 400 patients enrolled in OAK were retrospectively analysed for their PD-L1 expression using the 22C3 assay. These results were compared with the PD-L1 scores generated on the 400 tumours in the original SP142 analysis.
The investigators found that the vast majority (77 %) of SP142 PD-L1–negative patients were also PD-L1–negative according to the 22C3 assay. Atezolizumab significantly improved survival in patients with PD-L1–negative tumours according to either assay (HRs, 0.55 and 0.61 with SP142 and 22C3, respectively). Patients whose tumours were defined as PD-L1–negative by both assays showed improved OS with atezolizumab compared to docetaxel (9.9 vs. 7.7 months; HR, 0.63; p = 0.0347). This OS benefit was consistent with the overall OAK trial results.
Novel blood-based assay for tumour mutational burden
Another analysis based on the OAK and POPLAR studies demonstrated that tumour mutational burden (TMB) can be measured in blood (bTMB), and that bTMB is associated with improved PFS from immune checkpoint inhibitor therapy [5]. Gandara et al. tested a novel blood-based assay for the measurement of bTMB and evaluated the association between bTMB and atezolizumab efficacy. TMB, when measured in tumour tissue, was previously shown to correlate with atezolizumab efficacy in NSCLC [6], but as tissue is inadequate for molecular testing in approximately one third of newly diagnosed NSCLC patients, alternative sources of diagnostic material are called for.
Plasma samples from POPLAR and OAK were retrospectively assessed for bTMB using a next generation sequencing assay based on 394 genes. Two hundred eleven of 273 samples from POPLAR and 583 of 797 samples from OAK were biomarker-evaluable and constituted the biomarker-evaluable population (BEP) for the study. Circulating cell-free DNA was extracted. All base substitutions with a ≥ 0.5 % allele frequency were counted, while germline polymorphisms and predicted driver mutations were removed. In their entirety, these results constituted the bTMB score.
Enrichment for both PFS and OS was observed in the POPLAR study at several levels of bTMB, but the assay performed best at the bTMB ≥ 16 level (HR for PFS, 0.57; HR for OS, 0.56). Based on these data, bTMB ≥ 16 was selected for the confirmatory analysis in the OAK trial. The bTMB ≥ 16 population accounted for 27 % of the BEP.
Higher bBMT predicts greater PFS benefit
After there had been no overall PFS improvement in the OAK trial, the bTMB ≥ 16 subgroup showed a PFS benefit with atezolizumab compared to docetaxel. However, no prognostic effect was observed, as docetaxel-treated patients in the bTMB ≥ 16 group did not experience any PFS improvement compared to those who had bTMB < 16. For OS, the hazard ratios favouring atezolizumab over docetaxel were similar across the bTMB ≥ 16 and < 16 groups (0.64 and 0.65, respectively). This result might reflect the impact of subsequent therapies post progression, particularly in the docetaxel cohort. Median OS for the bTMB ≥ 16 subgroup was 13.5 vs. 6.8 with atezolizumab and docetaxel, respectively. An exploratory analysis found a linear increase of PFS benefits with higher bTMB cut-points (Figure 2). For OS, this effect was somewhat mitigated. According to an analysis of the correlation between baseline characteristics and bTMB subgroups, higher bTMB scores were associated with smoking. There was also a possible association between bTMB and clinical tumour volume, as measured by the sum of longest diameters or number of metastatic sites.
The comparison of a tissue-based TMB assay with bTMB yielded a positive percentage agreement (PPA) of 64 % and a negative percentage agreement (NPA) of 88 % (Spearman correlation, 0.59). The relatively low PPA might have been influenced by factors such as tumour heterogeneity, different computational methodologies, and different specimen acquisition times. When the same circulating tumour DNA was used, PPA and NPA were improved and supported use of the bTMB ≥ 16 cut-point. Another issue related to the potential correlation between bTMB ≥ 16 and PD-L1 expression. Here, the overlap was not significant, with only 30 patients out of 229 showing both a bTMB ≥ 16 score and the highest level of PD-L1 expression as measured by IHC.
As the authors summarised, bTMB might be particularly useful for the up to 30 % of patients who lack sufficient tissue for molecular testing. Prospective studies in the first-line setting using the bTMB assay are ongoing.
Figure 2: Incremental PFS benefit of atezolizumab with increasing bTMB cut-points in OAK with increasing bTMB cut-points in OAK
Three-year follow-up of CheckMate 017 and 057
The anti-PD-1 antibody nivolumab has been approved in many countries for the treatment of patients with advanced NSCLC and disease progression during or after chemotherapy based on the global, randomised, open-label CheckMate 017 [7] and CheckMate 057 [8] phase III trials. CheckMate 017 investigated nivolumab in patients with squamous histology, while CheckMate 057 included patients with non-squamous NSCLC. Both trials showed significantly improved OS and a favourable safety profile in the nivolumab arm as compared to docetaxel. Felip et al. presented the updated efficacy and safety data from CheckMate 017 and CheckMate 057 based on at least three years of follow-up [9].
Nivolumab continued to demonstrate long-term benefits, with the OS and PFS curves plateauing in both trials. The 3-year OS rates in CheckMate 017 were 16 % versus 6 % with nivolumab and docetaxel, respectively. In CheckMate 057, these were 18 % versus 9 %. As is known, PD-L1 expression predicted the OS benefit in the non-squamous group included in CheckMate 057, while this effect was less pronounced in the CheckMate 017 population with squamous histology. Three-year PFS rates were 12 % versus not calculable in CheckMate 017, and 10 % versus < 1 % in CheckMate 057.
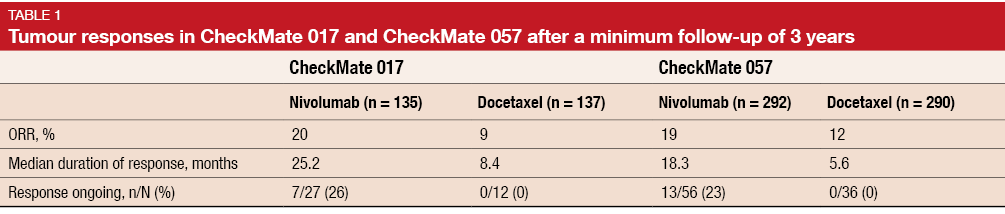
Among responders, patients treated with nivolumab experienced longer median duration of response than those in the docetaxel arm. Twenty-six percent and 23 % of patients in CheckMate 017 and CheckMate 057, respectively, who responded to nivolumab, had ongoing tumour responses (Table 1). No ongoing responses were observed in the docetaxel arms of the two trials. The long-term follow-up showed no new safety signals for nivolumab, and rates of treatment-related AEs were similar to those seen in the past.
Data on nivolumab in the elderly
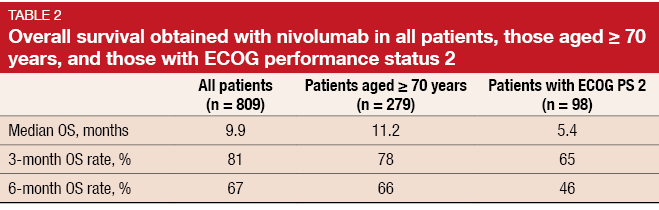
Preliminary results from the large ongoing CheckMate 171 study support nivolumab as a therapeutic option in previously treated patients with advanced, squamous NSCLC, including those aged ≥ 70 years or with an ECOG performance status (PS) of 2 [10]. Most lung cancer patients are diagnosed at an advanced age and therefore frequently present with comorbidities. However, data on therapeutic options in these patients are limited, as they are usually under-represented in randomised clinical trials. The single-arm, phase II CheckMate 171 study is exploring safety and survival outcomes in heavily pre-treated patients (n = 809) who received nivolumab monotherapy after progression on platinum-based chemotherapy, including patients aged ≥ 70 years (n = 279) and those with ECOG PS 2 (n = 98).
The analysis showed that estimated median OS as well as OS rates at 3 and 6 months were comparable across the overall population and patients aged ≥ 70 years (Table 2). Patients with ECOG PS 2 experienced slightly poorer results. PR rates at week 9 were 14 %, 14 % and 11 % for the overall population, patients aged ≥ 70 years, and those with ECOG PS 2, respectively. The safety profile of nivolumab was comparable across these three groups, including rates of grade 3/4 treatment-related AEs, all-grade AEs, and AEs leading to discontinuation.
How long should nivolumab be administered?
Optimal duration of treatment with PD-(L)1 inhibitors remains an important question. While the majority of nivolumab data are based on treatment until disease progression or unacceptable toxicity, findings from the phase I CheckMate 003 study suggest that approximately two years of nivolumab monotherapy are sufficient for long-term clinical benefit in patients with previously treated NSCLC [11]. CheckMate 153 was the first randomised study to evaluate duration of treatment with a PD-(L)1 inhibitor [12]. It compared continuous administration of nivolumab versus nivolumab treatment limited to one year. Patients with advanced or metastatic NSCLC of squamous or non-squamous histology who had at least one prior systemic therapy were eligible. ECOG performance status of 0-2 was allowed, as well as treated CNS metastases. This was a heavily pre-treated cohort; a quarter in each arm had received at least three prior therapies, and one third had received two therapies.
All of the patients (n = 220) underwent treatment with the standard dose of nivolumab (3 mg/kg every 2 weeks) for one year. After that, they were randomised to either continuous nivolumab or treatment discontinuation, with the opportunity to receive retreatment at progression. Only patients who had achieved disease control (i.e., complete response [CR], PR, SD) at the time of randomisation were included in the efficacy analysis. This applied to 76 patients in the continuous arm and 87 in the discontinuation arm.
Findings support continuous treatment
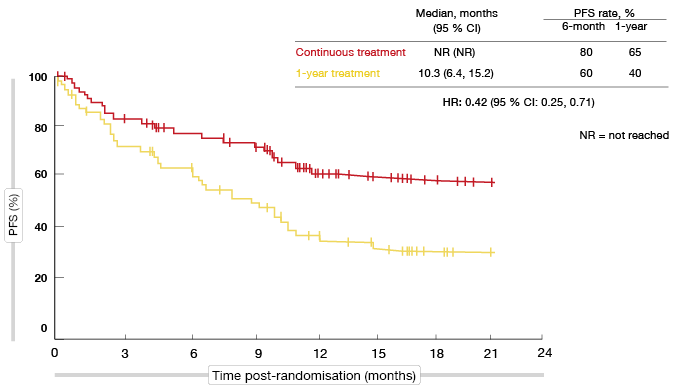
Within this group, patients derived significantly greater benefit from continuous nivolumab with respect to PFS from randomisation (not reached vs. 10.3 months; HR, 0.42; Figure 3). At one year, PFS rates were 65 % vs. 40 %. Furthermore, continuous nivolumab showed greater activity independent of response status at the time of randomisation: for patients with CR or PR, median PFS was not reached vs. 10.6 months (HR, 0.45), and for those with SD, not reached vs. 9.6 months (HR, 0.44). The multivariate analysis favoured continuous nivolumab (HR, 0.43) even after adjustment for gender, histology, best overall response, and PD-L1 status. OS was longer for continuous nivolumab, although not to a statistically significant extent.
Among patients who were randomised to treatment discontinuation, 43 experienced disease progression thereafter. Thirty-four of these were retreated with nivolumab. Median duration of retreatment was 3.8 months (range, 0.1–17.5 months). Most patients showed increases in target lesion size, although some experienced treatment benefits. Regarding safety after randomisation, there was a generally higher incidence of treatment-related (serious) AEs in the continuous treatment arm compared to the 1-year treatment arm. Few new-onset events occurred after one year. No treatment-related deaths were reported in either of the trial arms, and no new safety signals emerged in the overall cohort.
Figure 3: Continuous nivolumab administration vs. discontinuation after 1 year: progression-free survival
Updated results of KEYNOTE-021
Cohort G of the KEYNOTE-021 study was an open-label, randomised phase II trial investigating the combination of the anti-PD-1 antibody pembrolizumab with pemetrexed/ carboplatin chemotherapy compared to pemetrexed/ carboplatin alone. Patients with previously untreated advanced non-squamous NSCLC were enrolled. According to the primary analysis conducted after a median follow-up of 10.6 months, the pembrolizumab-based combination conferred significant improvements with regard to ORR (55 % vs. 29 %; p = 0.0016) and PFS (HR, 0.53; p = 0.010) [13]. At that time, HR for OS was 0.90. An updated analysis presented at the ASCO Congress 2017 showed that the ORR and PFS benefits were maintained, while the mortality risk had decreased (HR for OS, 0.69; p = 0.13) [14]. Both the primary and secondary analysis yielded a manageable safety profile of the combination.
At the ESMO 2017 Congress, Borghaei et al. presented updated findings after a median follow-up of 18.7 months [15]. Again, the significant improvements in ORR and PFS were maintained. ORR was 56.7 % vs. 31.7 %. As compared with the pre-specified analysis, three additional responses had been observed, one in the experimental arm and two in the control arm. In each group, one CR had developed. Median duration of response in either arm had not been reached; 50 % vs. 40 % of patients showed ongoing responses. PFS for the pembrolizumab and chemotherapy-only arms was 19.0 vs. 8.9 months, respectively (HR, 0.54). For OS, the incremental benefit continued to increase (HR, 0.59) despite the substantial proportion of patients in the control arm who had received anti-PD-(L)1 treatment inside and outside of the crossover (63 % of the intent-to-treat population). However, the OS difference was not statistically significant due to low patient numbers.
Likewise, changes in toxicity compared to the last update were limited. One additional AE had led to treatment discontinuation in each arm. Toxicity profiles were as anticipated. No grade 5 AEs with possible immune aetiology had occurred.
Prevalence and impact of hyper-progression
Hyper-progressive disease (HPD) in the context of immunotherapy has been described in 9 % of 131 advanced cancer patients treated with immunotherapy in early phase trials [16]. Lahmar et al. reported increases of > 50 % of tumour volume in 10 % of 89 NSCLC patients [17]. A retrospective study conducted at five French institutions assessed the prevalence of HPD, its prognostic value and its correlation with clinical characteristics in a large cohort of patients with advanced NSCLC who received immunotherapy [18]. Two CT scans were required before the start of immunotherapy and one during treatment; the interval between the baseline CT scan and the start of treatment had to be ≤ 6 weeks. CT scans were centrally assessed according to RECIST 1.1. Of 365 screened patients, 242 (66 %) were included.
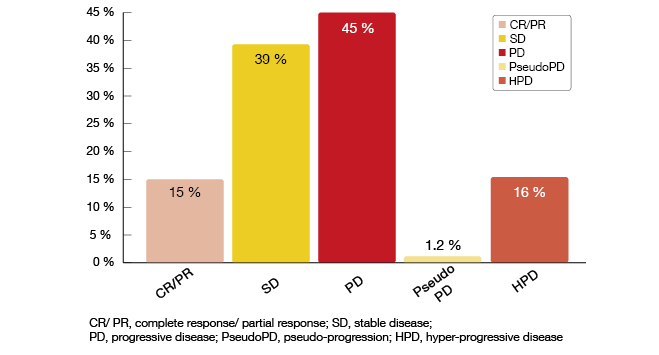
According to this analysis, the administration of immunotherapy accelerated tumour growth in 36 % of patients, while 64 % showed either regression or SD. In 40 cases (16 %), HPD occurred (Figure 4), which was defined as a > 50 % difference across the tumour growth rates before and after treatment initiation. Pseudo-progression took place in 1.2 %.
The analysis of clinical characteristics according to progression status yielded increased risk for HPD in the presence of more than two metastatic sites before the start of treatment. HPD was identified as a negative prognostic factor: for patients experiencing progression that was not HPD, median OS was 5.7 months, whereas those developing HPD showed a median OS of only 3.3 months (HR, 0.39; p = 0.011).
Figure 4: Response patterns in immunotherapy-treated lung cancer patients
References
- Paz-Ares L et al., PACIFIC: a double-blind, placebo-controlled phase III study of durvalumab after chemoradiation therapy in patients with stage III, locally advanced, unresectable NSCLC. ESMO 2017, abstract LBA1_PR
- Rittmeyer A et al., Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389(10066): 255-265
- Fehrenbacher L et al., Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387(10030): 1837-1846
- Gadgeel S et al., Clinical efficacy of atezolizumab in PD-L1 selected subgroups defined by SP142 and 22C3 IHC assays in 2L+ NSCLC: results from the randomised OAK trial. ESMO 2017, abstract 1296O
- Gandara DR et al., Blood-based biomarkers for cancer immunotherapy: tumour mutational burden in blood (bTMB) is associated with improved atezolizumab efficacy in 2L+ NSCLC (POPLAR and OAK). ESMO 2017, abstract 1295O
- Kowanetz M et al., Tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. WCLC 2016, abstract OA20.01
- Brahmer J et al., Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123-135
- Borghaei H et al., Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627-1639
- Felip E et al., Three-year follow-up from CheckMate 017/057: nivolumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. ESMO 2017, abstract 1301PD
- Popat S et al., Nivolumab in previously treated patients with metastatic squamous NSCLC: results of a European, single-arm, phase 2 trial (CheckMate 171) including patients aged ≥ 70 years or with poor performance status. ESMO 2017, abstract 1303P
- Brahmer J et al., AACR Annual Meeting 2017, abstract 8356
- Spigel DR et al., CheckMate 153: randomised results of continuous vs. 1-year fixed-duration nivolumab in patients with advanced non-small cell lung cancer. ESMO 2016, abstract 1297O
- Langer CJ et al., Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497-1508
- Papadimitrakopoulou P et al., First-line carboplatin and pemetrexed (CP) with or without pembrolizumab (pembro) for advanced nonsquamous NSCLC: updated results of KEYNOTE-021 cohort G. J Clin Oncol 2017; 35 (suppl; abstr 9094)
- Borghaei H et al., Updated results from KEYNOTE-021 cohort G: a randomised, phase 2 study of pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small-cell lung cancer. ESMO 2017, abstract LBA49
- Champiat S et al., Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017; 23(8): 1920-1928
- Lahmar J et al., Immune checkpoint inhibitors induce paradoxical progression in a subset of non-small cell lung cancer (NSCLC). Ann Oncol 2016; 27 (suppl_6): 1222P
- Ferrara R et al., Hyperprogressive disease (HPD) is frequent in non-small cell lung cancer (NSCLC) patients treated with anti PD-1/PD-L1 monoclonal antibodies (IO) and predicts poor survival. ESMO 2017, abstract 1306PD