Determinants of treatment success in small-cell lung cancer
Predictive characteristics in CASPIAN …
The randomized, controlled, open-label, phase III CASPIAN trial has assessed first-line treatment with durvalumab ± tremelimumab plus platinum/etoposide (EP) compared to EP alone in patients with extensive-stage small-cell lung cancer (ES-SCLC). Durvalumab plus EP significantly improved OS compared to EP alone (HR, 0.73; p = 0.0047) [1]. This benefit was maintained after more than 2 years of median follow-up (12.9 vs. 10.5 months; HR, 0.75; p = 0.0032) [2]. At 24 months, 22.2 % vs. 14.4 % of patients were alive. Exploratory analyses were conducted to identify clinical characteristics that might predict outcomes in patients deriving long-term benefit, as well as the relationship between tissue tumor mutational burden (tTMB) and efficacy [3]. PFS ≥ 12 months was used as a preliminary threshold to identify potential predictive parameters in treated patients.
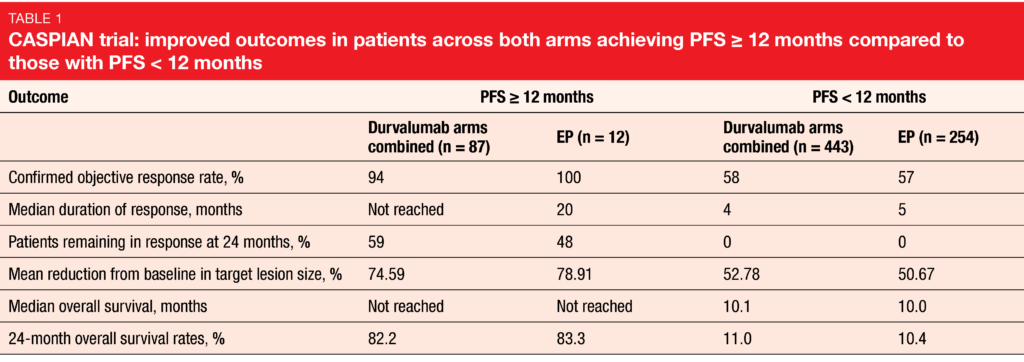
In the durvalumab plus EP group, 45 of 265 patients (17.0 %) achieved PFS ≥ 12 months, and in the durvalumab plus tremelimumab plus EP arm, 42 of 266 patients (15.8 %). For both immunotherapy arms combined, the percentage of patients achieving PFS ≥ 12 months (87/531, 16.4 %) was more than 3 times higher than that in the EP arm (12/266, 4.5 %). Patients with PFS ≥ 12 months in all arms had improved ORR, duration of response, depth of response and OS compared to the subgroups with PFS < 12 months (Table 1). The 2-year OS rates exceeded 75 %, which denotes an exceptional long-term benefit.
However, no unique clinical characteristics identified those who achieved long-term benefit. Across all treatment arms, patients with PFS ≥ 12 months showed a higher incidence of traditional favorable prognostic factors such as ECOG performance status 0 and lack of brain or liver metastases, although poor prognostic factors were also present in a meaningful percentage of patients. The groups with PFS ≥ 12 months and < 12 months did not differ with regard to use of cisplatin or overall chemotherapy exposure. Also, tTMB was not shown to predict OS improvement with durvalumab ± tremelimumab plus EP vs. EP alone at any cutoff or as a continuous variable. Despite greater exposure to durvalumab and numerically higher rates of immune-mediated AEs in the PFS ≥ 12 subgroup, rates of grade 3/4 AEs were similar to those in the PFS < 12 subgroup, as were serious AEs and AEs leading to discontinuation. Further investigation into predictive factors for long-term benefit with durvalumab is ongoing.
… and in IMpower133
Atezolizumab plus carboplatin/etoposide has been evaluated for the first-line treatment of ES-SCLC in the double-blind, placebo-controlled, phase III IMpower133 trial. Compared to placebo plus carboplatin/etoposide, the immunotherapy-based regimen gave rise to improvements in OS (12.3 vs. 10.3 months; HR, 0.70; p = 0.007) and PFS (5.2 vs. 4.3 months; HR, 0.77; p = 0.02) [4]. Additional follow-up showed persistent OS benefit in the experimental arm with increased survival rates at 12, 18 and 24 months, establishing the IMpower133 regimen as a new standard of care [5]. As data are limited regarding the characteristics of patients who experienced long-term survival, Liu et al. presented exploratory analyses to characterize long-term survivors (LTS) defined as patients who lived ≥ 18 months since randomization [6].
The comparison across the study arms of IMpower133 showed that a greater proportion of LTS was treated with atezolizumab plus chemotherapy (61 of 182 patients, 33.5 %) than with placebo plus chemotherapy (39 of 191 patients, 20.4 %). For each of the patient characteristics (i.e., sex, age, ECOG performance status), more patients in the LTS group had received atezolizumab plus chemotherapy than chemotherapy alone. The same applied to disease characteristics (i.e., number of metastatic sites, presence of liver metastases or brain metastases, elevated LDH levels, greater sum of longest diameters of target lesions) that are typically associated with greater disease burden. With respect to each of these, LTS were more likely to have received atezolizumab rather than placebo.
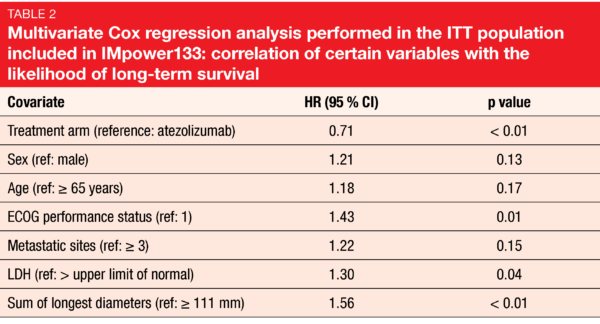
According to the biomarker analysis, high blood-based TMB and PD-L1 expression using various cutoffs did not correlate with the likelihood of long-term survival. Therefore, neither TMB nor PD-L1 status appeared to have utility in patient selection. In the multivariate Cox regression analysis, only worse performance status, elevated LDH levels and highest sum of longest diameters were confirmed as poor prognostic variables (Table 2). The impact of atezolizumab treatment on OS was more pronounced after adjustment for other covariates in the multivariate model, with a significant HR of 0.71. As the authors concluded, these exploratory analyses suggest that patients with ES-SCLC can derive benefit from the addition of atezolizumab plus chemotherapy regardless of the patient and disease characteristics evaluated, confirming atezolizumab plus carboplatin/etoposide as a standard of care for patients with untreated ES-SCLC.
STIMULI: consolidation immunotherapy
In patients with limited-stage SCLC (LS-SCLC), chemoradiotherapy (CRT) followed by prophylactic cranial irradiation (PCI) is the standard radical strategy. The global, randomized phase II ETOP/IFCT 4-12 STIMULI trial aimed to demonstrate superiority of nivolumab plus ipilimumab as consolidation treatment in patients with LS-SCLC (stage I-IIIB) who had not progressed after CRT and PCI [7]. The induction phase comprised nivolumab 1 mg/kg and ipilimumab 3 mg/kg Q3W for 4 cycles and was followed by maintenance nivolumab 240 mg Q2W for a maximum of 12 months. Patients in the control arm did not receive any further treatment. A total of 153 patients were randomized to nivolumab plus ipilimumab (n = 78) or observation (n = 75). For administrative reasons, the accrual was closed prematurely. Also, there was an unexpectedly low rate of identification of LS-SCLC cases and a high attrition rate (i.e. percentage of patients unable to complete CRT and PCI). Due to all this, the primary outcome was eventually defined as PFS after the original design had included a coprimary endpoint.
The STIMULI trial did not meet its primary endpoint (median PFS, 10.7 vs. 14.5 months for consolidation and observation, respectively; HR, 1.02; p = 0.93). Median time to treatment discontinuation in the experimental arm was as short as 1.7 months, and at 12 months, only 15.6 % of patients were still receiving treatment. This potentially explains the similar course of the curves, which crossed several times.
Benefits in certain subgroups
According to the subgroup analysis, patients with an ECOG performance status of 1 and those who received twice daily radiotherapy fractions appeared to derive a PFS benefit from nivolumab plus ipilimumab (HRs, 0.67 and 0.63, respectively). OS had not been reached in the experimental arm at the time of analysis and was 31.6 months in the control arm; this difference was not significant (HR, 1.06; p = 0.83). Again, benefits were seen for patients with ECOG PS 1 (HR, 0.44) and twice daily radiotherapy (0.41), as well as female patients (HR, 0.34).
Both treatment arms demonstrated the same pattern of progression. In the nivolumab plus ipilimumab arm, most treatment failures were due to toxicity, while in the control arm, disease progression constituted the most common reason for treatment failure. Treatment-related grade 3 to 5 AEs occurred in 51 % vs. 25 %, and most of these (49 %) led to treatment discontinuation in the experimental arm. The most frequent any-cause AEs included fatigue, anorexia, diarrhea, vomiting, pneumonitis, nausea, and cough.
In their conclusion, the authors noted that the short period on active treatment related to toxicity and treatment discontinuation has certainly impacted the efficacy results of the STIMULI trial. A longer follow-up will allow for the exploration of a possible late effect of immunotherapy consolidation on survival that was already apparent after the current short follow-up. Also, exploratory translational work is ongoing to identify biomarker-defined subgroups that could benefit from the consolidation immunotherapy treatment.
REFERENCES
- Paz-Ares L et al., Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019; 394: 1929-1939
- Paz-Ares L et al., Durvalumab ± tremelimumab + platinum-etoposide in first-line extensive-stage SCLC: updated results from the phase 3 CASPIAN study. J Clin Oncol 38: 2020 (suppl; abstr 9002)
- Goldman JW et al., Durvalumab ± tremelimumab + platinum-etoposide in 1L ES-SCLC: characterisation of long-term clinical benefit and tumour mutational burden in CASPIAN. ESMO 2020, LBA86
- Horn L et al., First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379(23): 2220-2229
- Reck M et al., IMpower133: updated overall survival analysis of first-line atezolizumab + carboplatin + etoposide in extensive-stage SCLC. Ann Oncol 2019; 30(suppl_5): v710-v717
- Liu SV et al., IMpower133: characterization of long-term survivors treated with first line chemotherapy ± atezolizumab in extensive-stage small cell lung cancer ESMO 2020, 1781MO
- Peters S et al., Consolidation nivolumab and ipilimumab vs observation in limited stage SCLC after chemo-radiotherapy – results from the randomized phase II ETOP/IFCT 4-12 STIMULI trial. ESMO 2020, LBA84
© 2020 Springer-Verlag GmbH, Impressum